PPIIMoL
PPIIMoL
PPIIMoL is a Python module for PyMOL that automates the detection of polyproline II (PPII) helices in proteins. It identifies PPII-like φ/ψ angle patterns, screens for plausible non‑canonical Cα–H···O=C contacts, and provides one‑click visualization and export.
This tool was developed as part of a Bachelor's Thesis in Computer Engineering in collaboration with the Protein Structure, Dynamics and Interactions by NMR Group at the Instituto de Química‑Física “Blas Cabrera” (IQF‑CSIC). The module’s design and validation take as a primary reference the data and architectonic principles reported by Segura Rodríguez & Laurents (2024). see References and How to cite below.
Scientific background
Polyproline II (PPII) helices are extended, left‑handed motifs (≈3 residues/turn) typically enriched in glycine‑ and proline‑rich domains. Although common in several glycine‑rich bundles, they are often unannotated in PDB files. PPIIMoL automates their detection directly in PyMOL to improve speed and reproducibility.
Features
- 🔍 Automatic detection of PPII segments via phi/psi angle analysis.
- 🧬 Identification of Cα-H···O=C interactions relevant to structural stability.
- 📊 CSV export of detected segments and interactions.
- 🎨 Direct visualization in PyMOL with customizable color codes.
- 🖱️ Simple Tkinter-based GUI — no commands required; all actions are accessible via buttons.
Requirements
Installation
Option A — Single‑file download (simplest)
- Download `PPIIMoL.py` from the repository (see Repository below).
- In PyMOL:
run /full/path/to/PPIIMoL.py
Option B — Clone the repository (optional)
git clone https://github.com/silviaenma/PPIIMoL.git
Then in PyMOL:
run PPIIMoL/PPIIMoL.py
Optional: install as a plugin
- In PyMOL: Plugin → Plugin Manager → Install New Plugin → select `PPIIMoL.py` (or the whole folder) → restart PyMOL.
Usage (GUI)
Once loaded, PPIIMoL opens a Tkinter window with buttons to:
- Load PDB (or use an already‑loaded object),
- Detect PPII (scan φ/ψ windows and list segments),
- Scan Cα–H···O=C (optional geometric screening),
- Style / Colors (apply the chosen palette),
- Export (CSV reports; optional per‑segment PDBs).
Results are written to a date‑stamped folder; selections/objects are created in the PyMOL session and colored according to the chosen scheme.
Example (command line, optional)
# Load the module (either single file or cloned repo) run /full/path/to/PPIIMoL.py # or run PPIIMoL/PPIIMoL.py # Load a structure and (optionally) trigger detection fetch 3bog, async=0 ppii_detect()
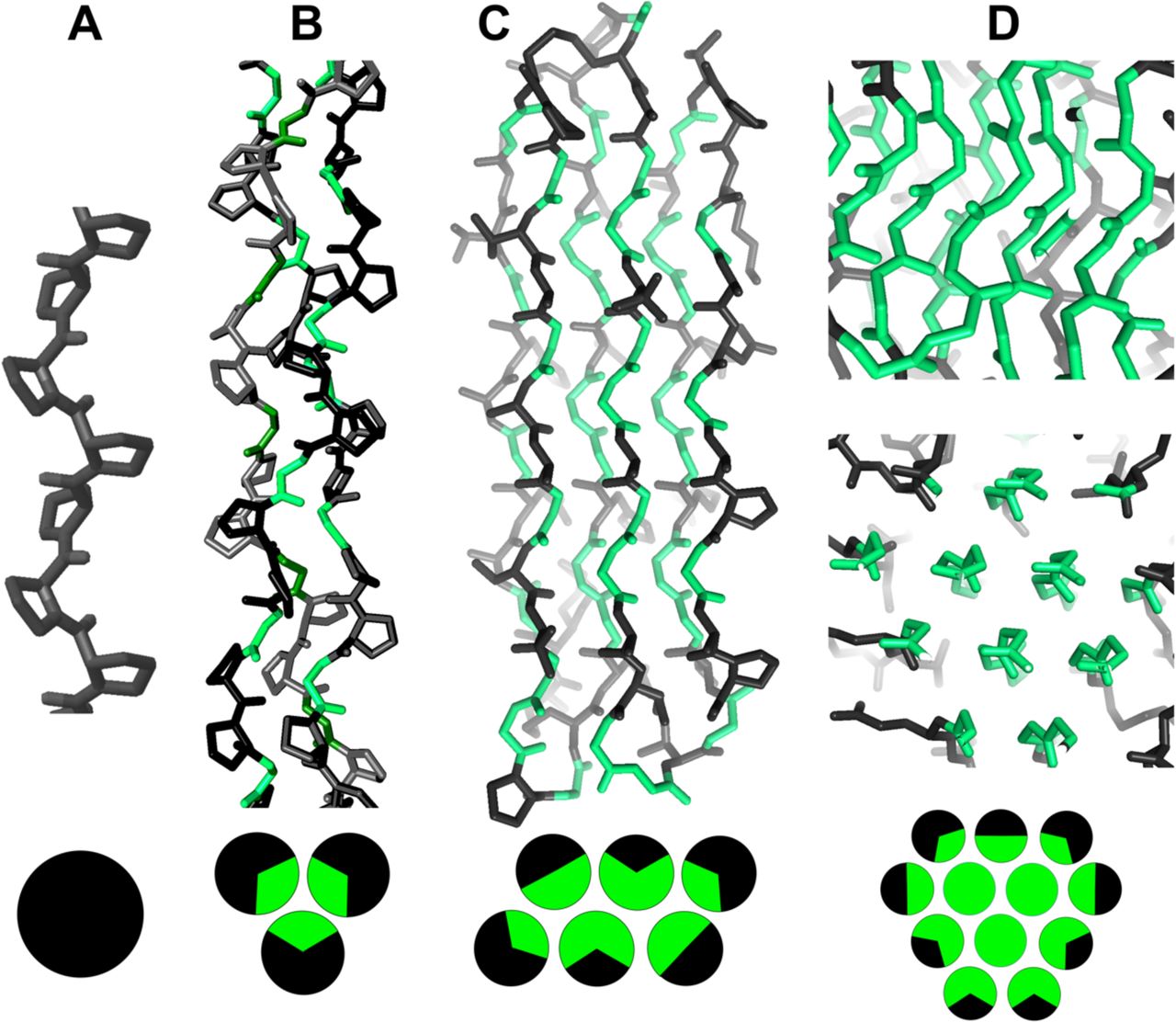
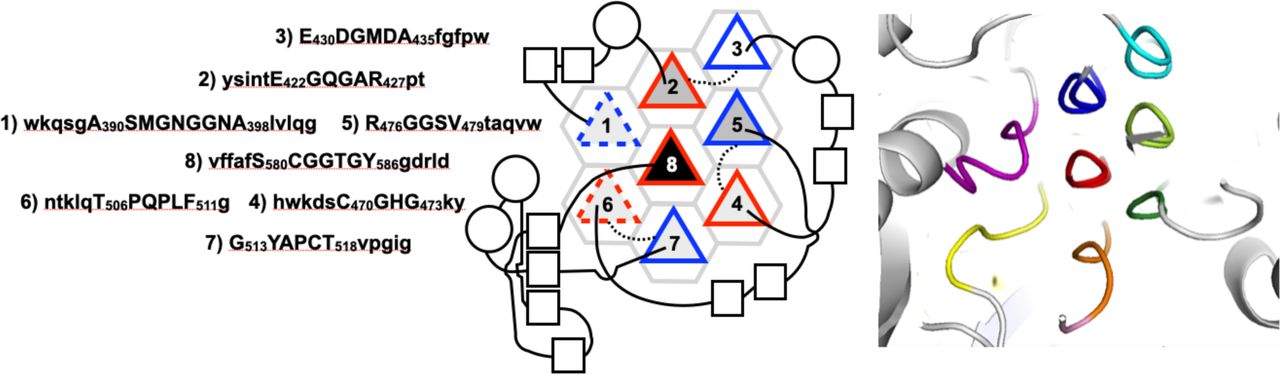
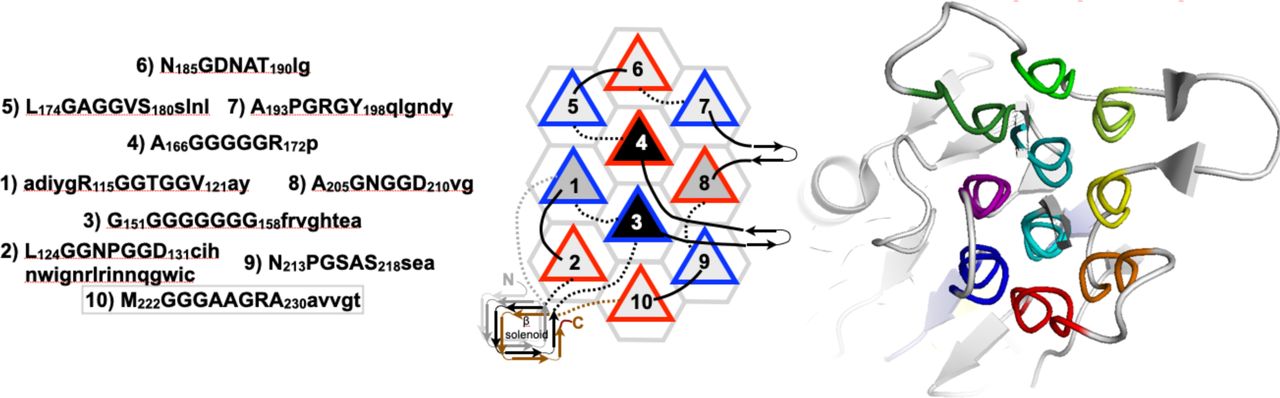
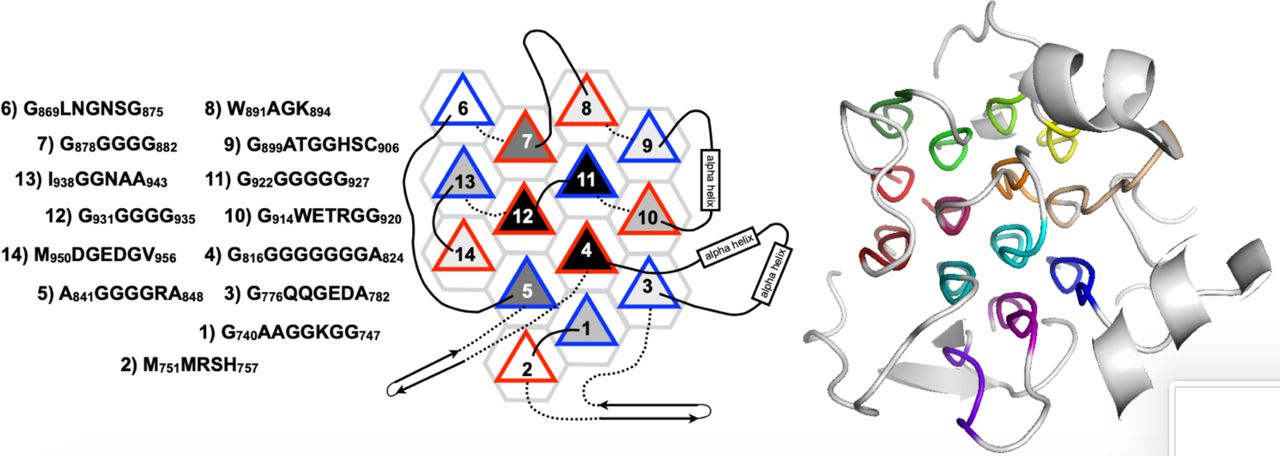
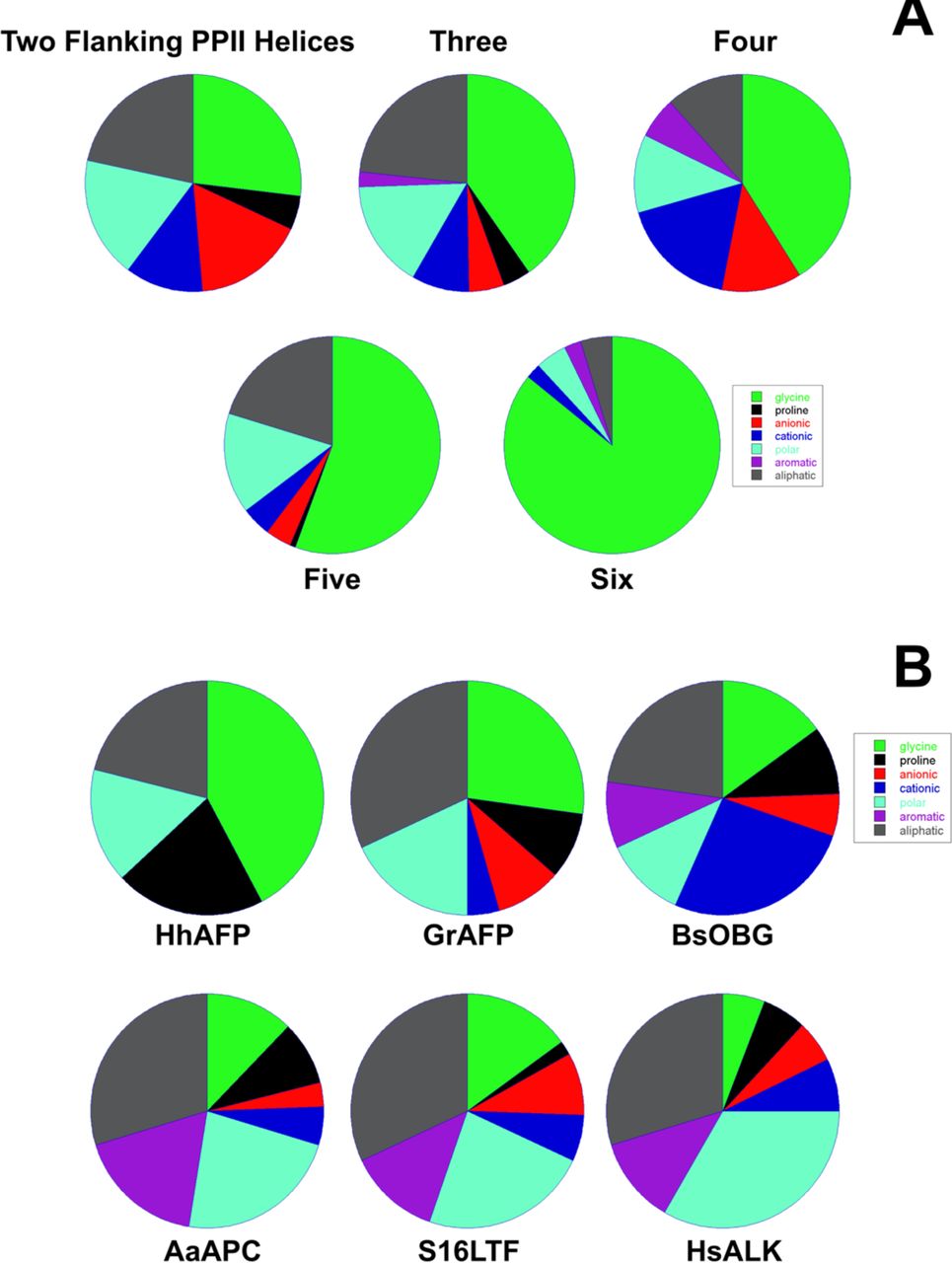
Legend (Fig. 1): (A) isolated PPII helix; (B) collagen triple helix; (C) double-layered sheets from (Pro–Gly–Gly)n with Pro outward and Gly inward; (D) 3D polyglycine II network. Reproduced with permission from Segura Rodríguez & Laurents (2024).
Reference figures
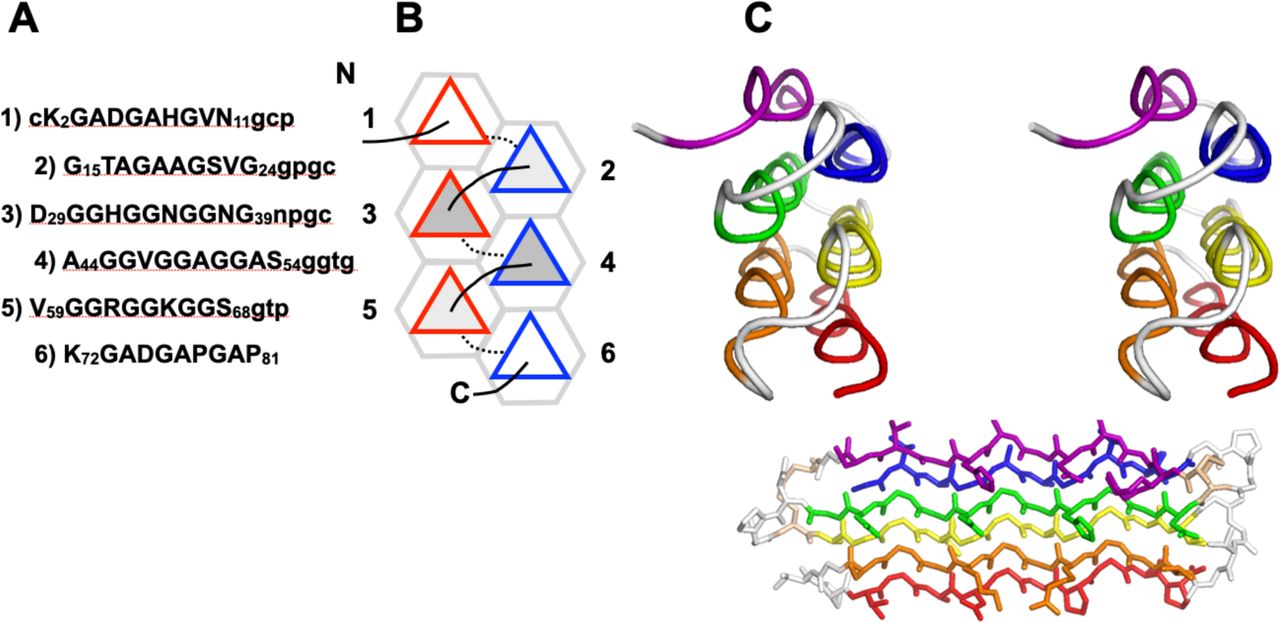
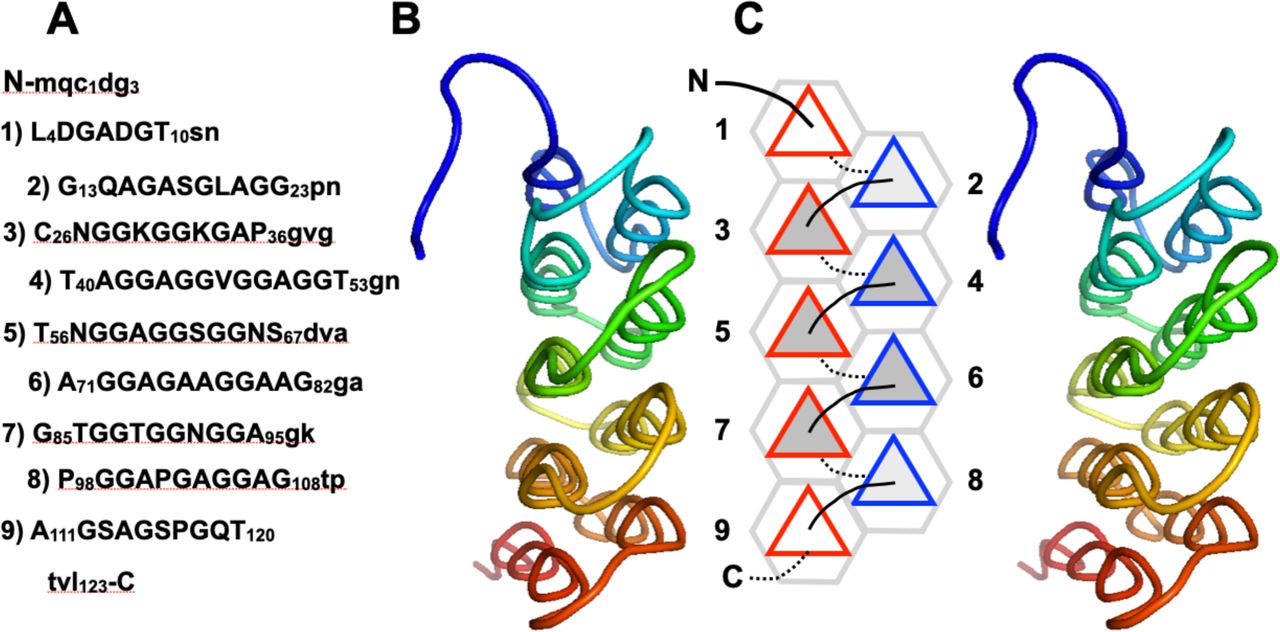
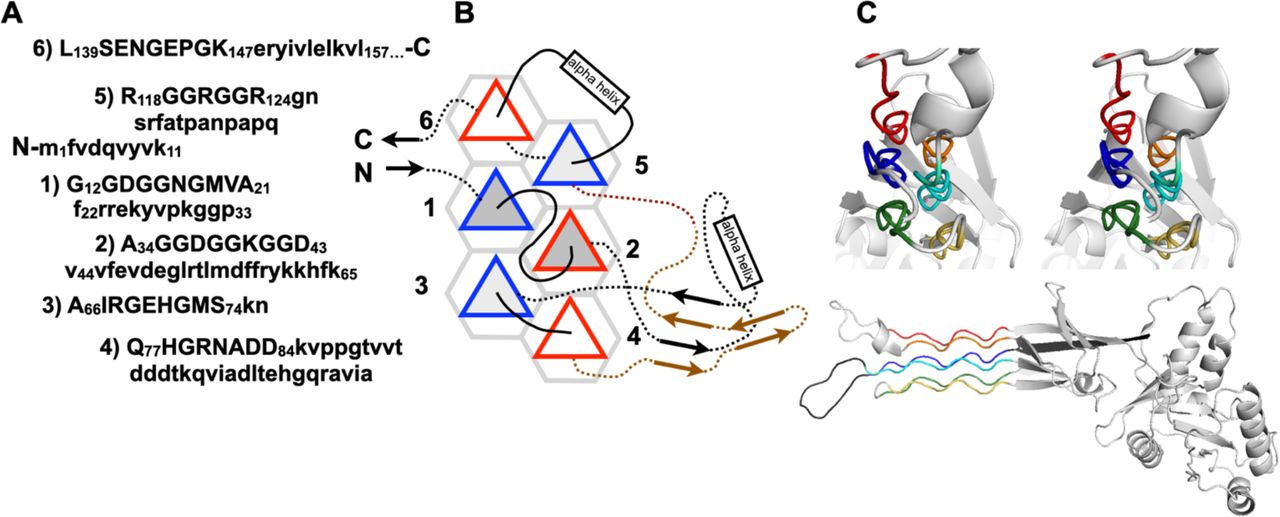
Below are reference figures illustrating PPII bundle organization and residue patterns, reproduced with permission from the authors of the cited work.
How to cite
If PPIIMoL is useful in your work, please cite both the software and the reference article:
- Software
- Rodríguez Fernández, S. E. (2025). PPIIMoL (version X.Y) [Computer software]. GitHub. https://github.com/silviaenma/PPIIMoL
- Reference article
- Segura Rodríguez, C. M., & Laurents, D. V. (2024). Architectonic principles of polyproline II helix bundle protein domains. Archives of Biochemistry and Biophysics, 741, 109981. https://doi.org/10.1016/j.abb.2024.109981
Repository
License
PPIIMoL is released under the GNU GPLv3.