PPIIMoL: Difference between revisions
| (32 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
= PPIIMoL = | = PPIIMoL = | ||
<span title="Small PPII helix icon"> | |||
[[File:PPIIMoL_icon.png|right|120px|alt=Small PPII helix icon|link=]] | |||
</span> | |||
'''PPIIMoL''' is a Python module for [[PyMOL]] that automates the detection of polyproline II (PPII) helices in proteins. It identifies PPII-like φ/ψ angle patterns, screens for plausible non-canonical Cα–H···O=C contacts, and provides one-click visualization and export. | '''PPIIMoL''' is a Python module for [[PyMOL]] that automates the detection of polyproline II (PPII) helices in proteins. It identifies PPII-like φ/ψ angle patterns, screens for plausible non-canonical Cα–H···O=C contacts, and provides one-click visualization and export. | ||
| Line 48: | Line 52: | ||
Results are written to a date-stamped folder; selections/objects are created in the PyMOL session and colored according to the chosen scheme. | Results are written to a date-stamped folder; selections/objects are created in the PyMOL session and colored according to the chosen scheme. | ||
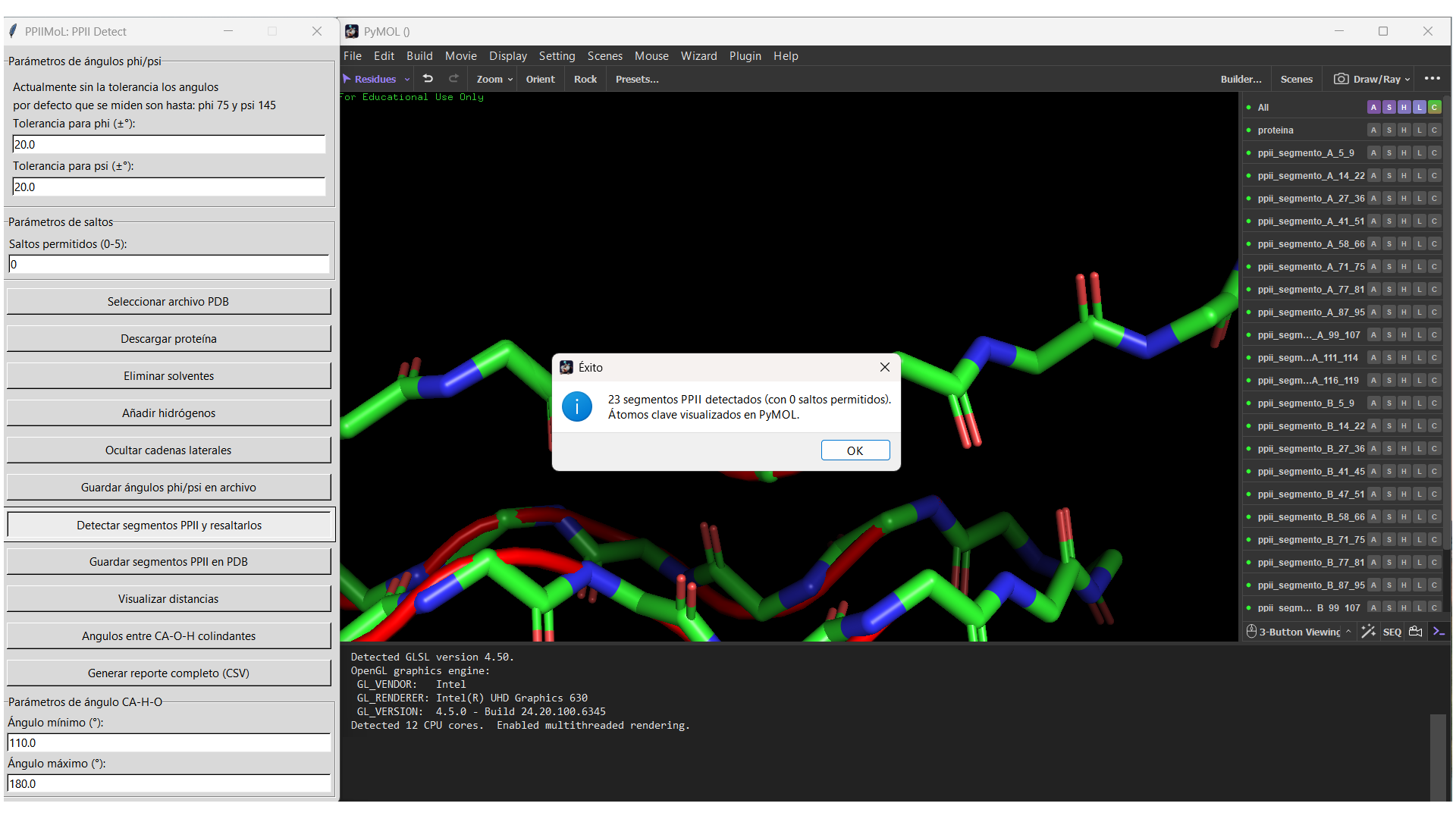
=== GUI snapshot === | |||
[[File:PPIIMoL_GUI.png|thumb|left|800px|alt=PyMOL window with the PPIIMoL pane and a success dialog after detecting PPII segments.|PPIIMoL graphical interface inside PyMOL. Main actions: Load PDB, Detect PPII, Scan Cα–H···O=C, Style/Colors, Export.]] | |||
<div style="clear:both;"></div> | |||
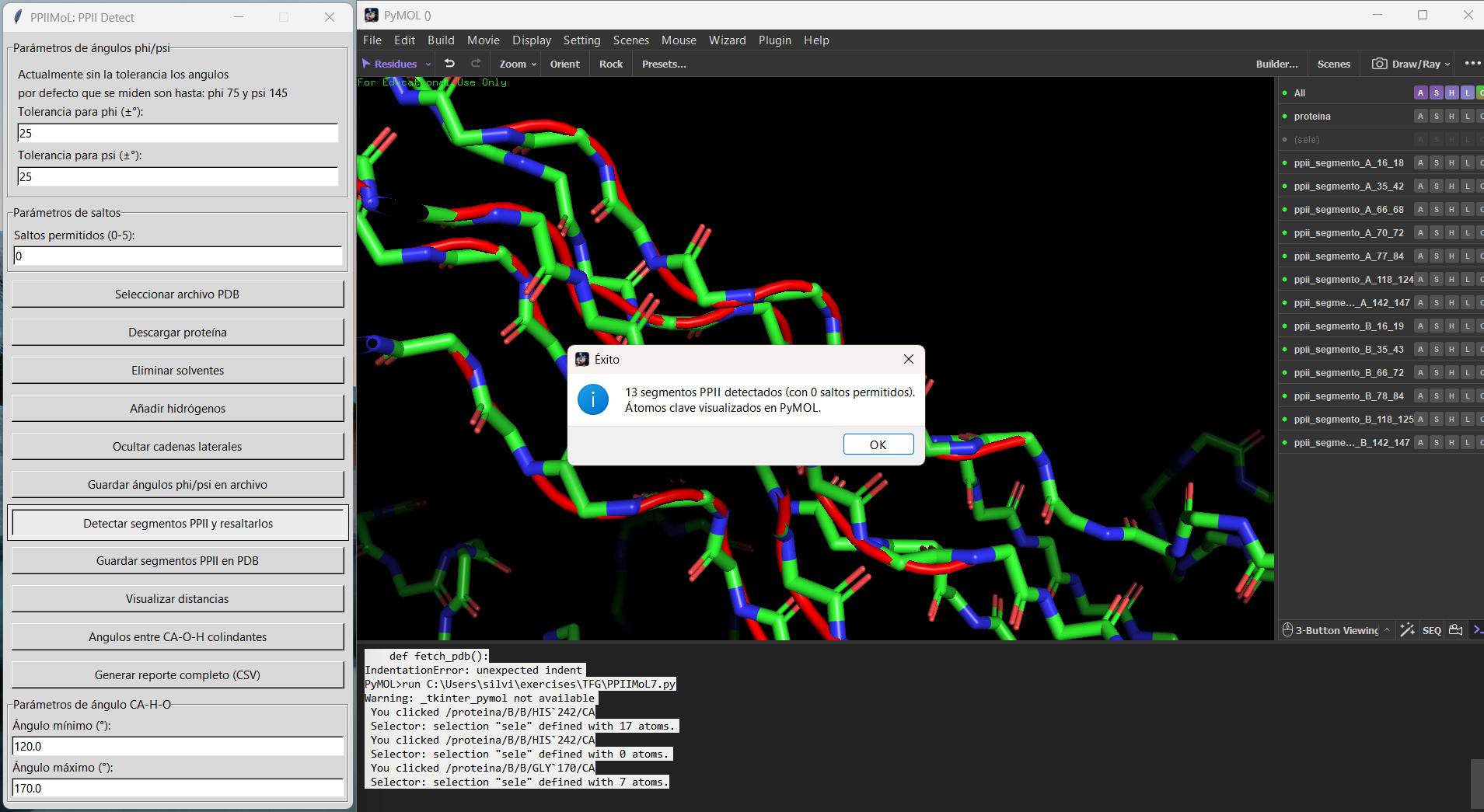
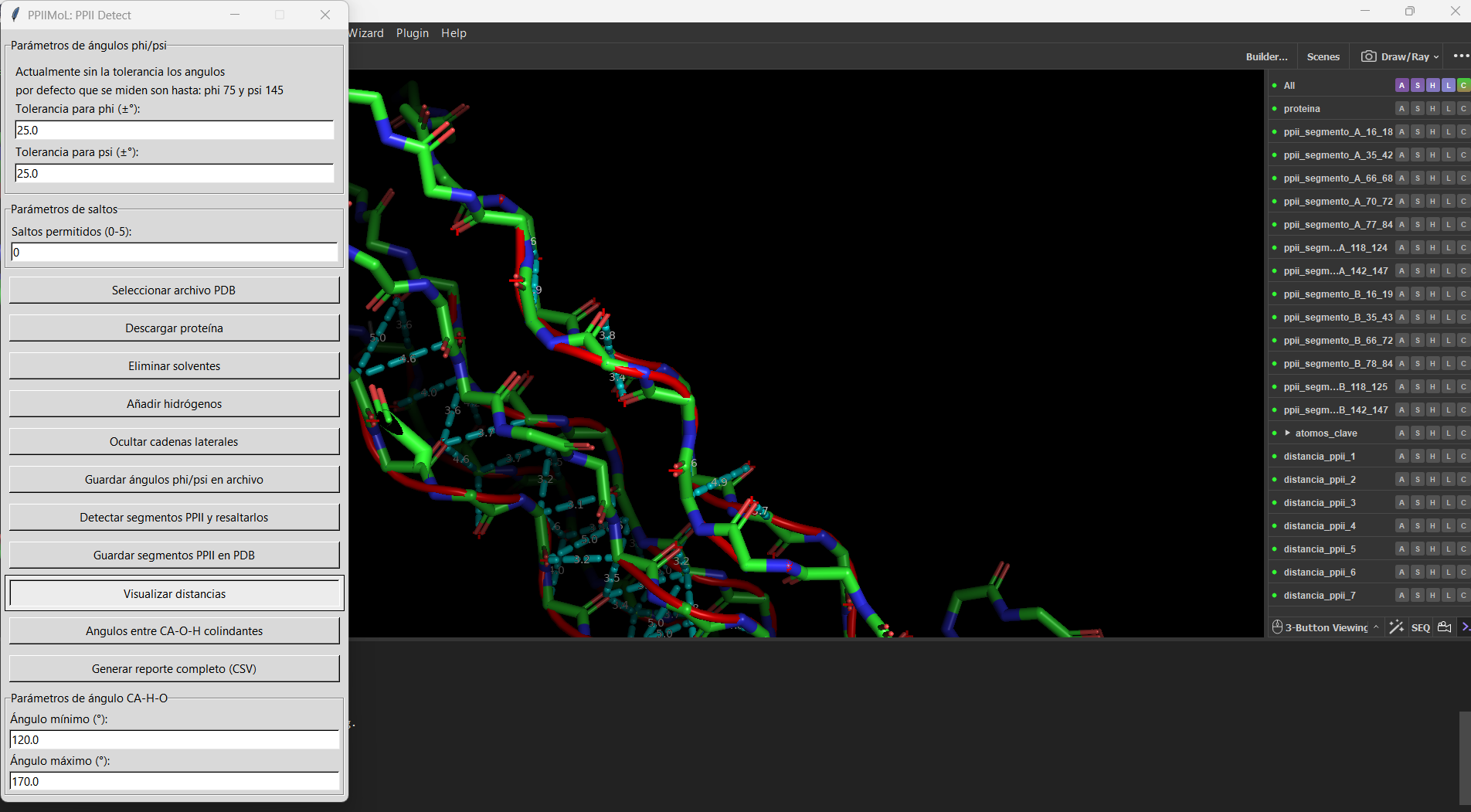
=== Examples === | |||
<gallery widths=520 heights=350 perrow=2> | |||
File:PPIIMoL_segments_1LNZ.png|Detected PPII segments colored by selection (example structure). | |||
File:PPIIMoL_distances_1LNZ.png|Distances and Cα–H···O=C angle labels between neighboring PPII segments. | |||
</gallery> | |||
== Example (command line, optional) == | == Example (command line, optional) == | ||
| Line 64: | Line 78: | ||
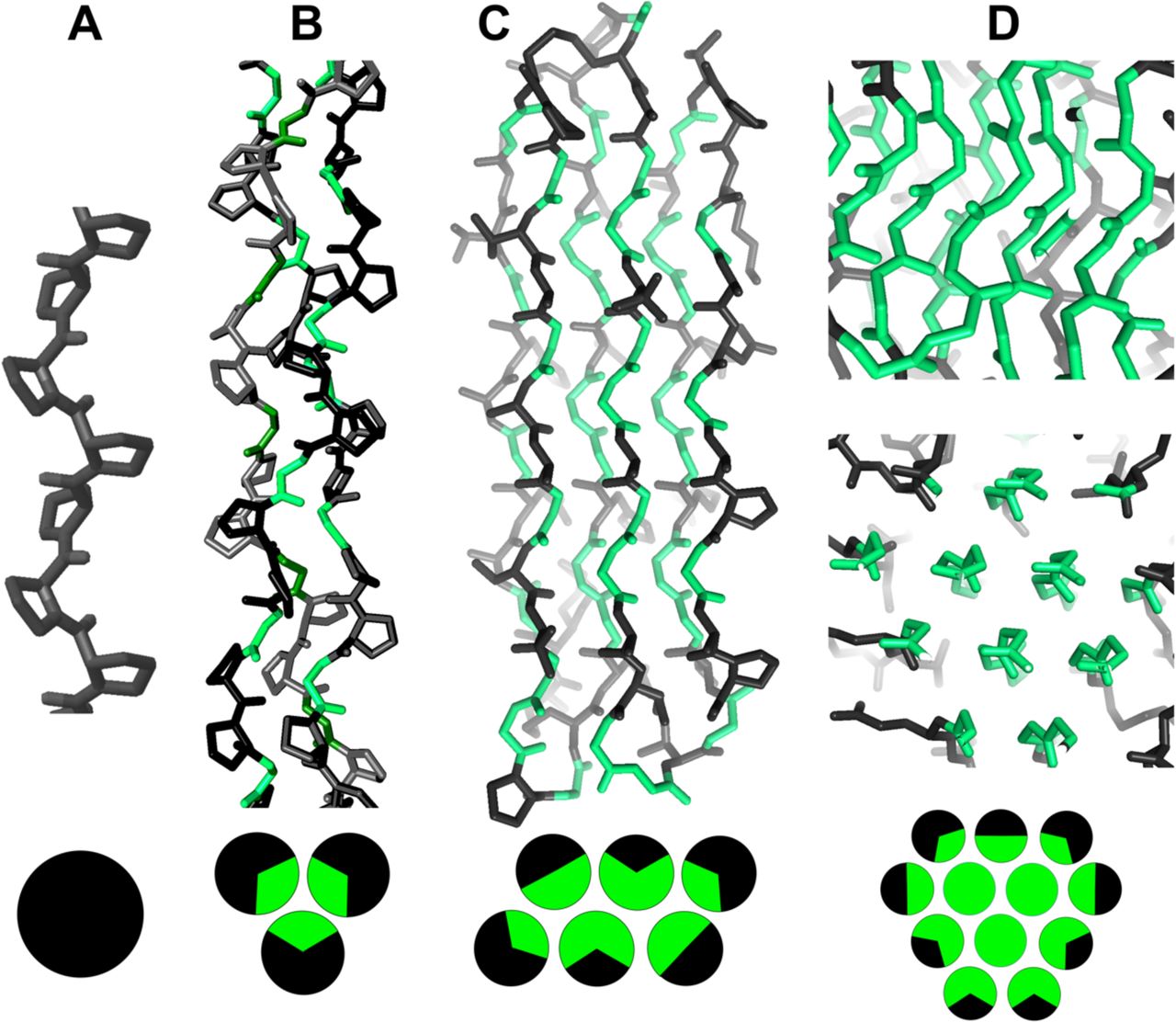
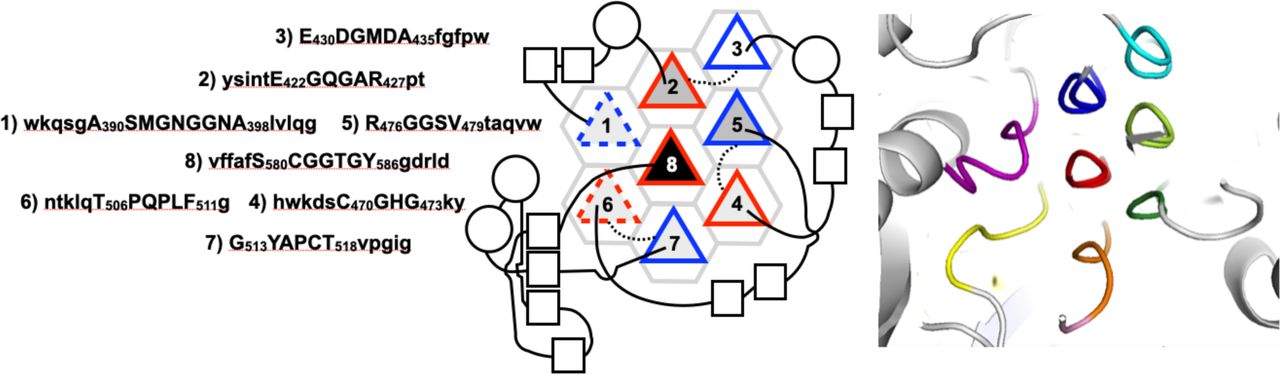
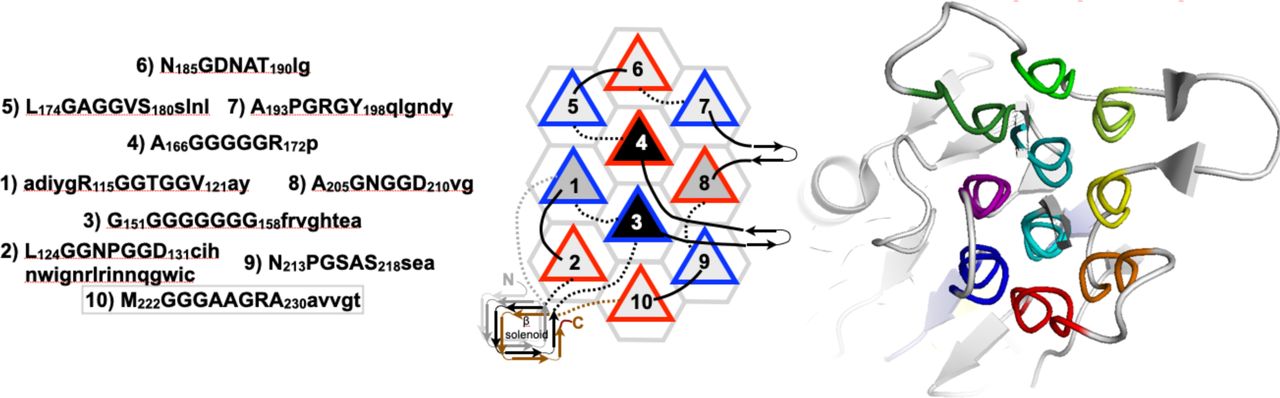
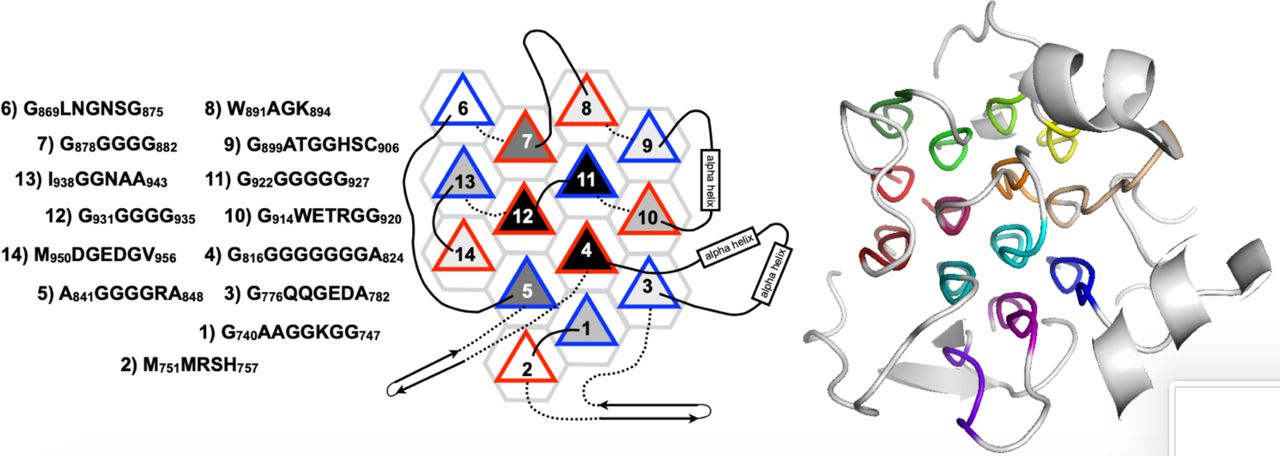
Below are reference figures illustrating PPII bundle organization and residue patterns, reproduced with permission from Segura Rodríguez & Laurents (2024). | Below are reference figures illustrating PPII bundle organization and residue patterns, reproduced with permission from Segura Rodríguez & Laurents (2024). | ||
<gallery widths= | <!-- Fila 1: Fig. 1 y Fig. 8 --> | ||
File:PPIIMoL Fig1.jpg|Figure 1. Overview of polyproline motifs.<br>Reproduced with permission from Segura Rodríguez & Laurents (2024) | <gallery widths=480 heights=640 perrow=2> <!-- añade mode=packed si tu wiki lo soporta --> | ||
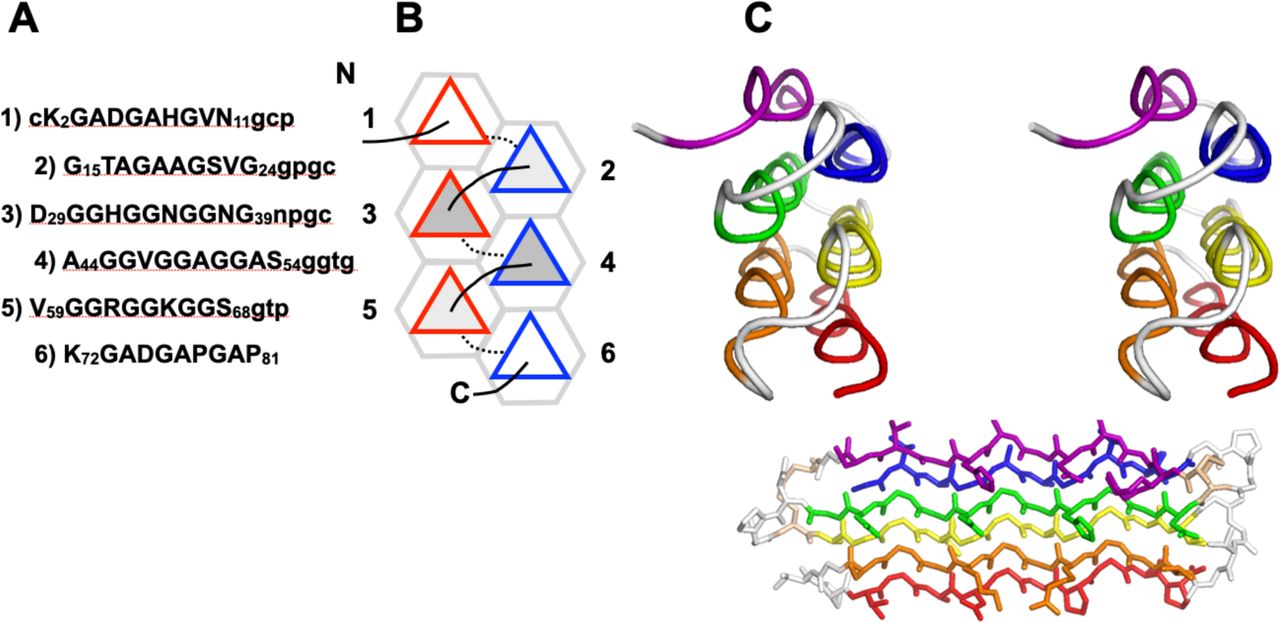
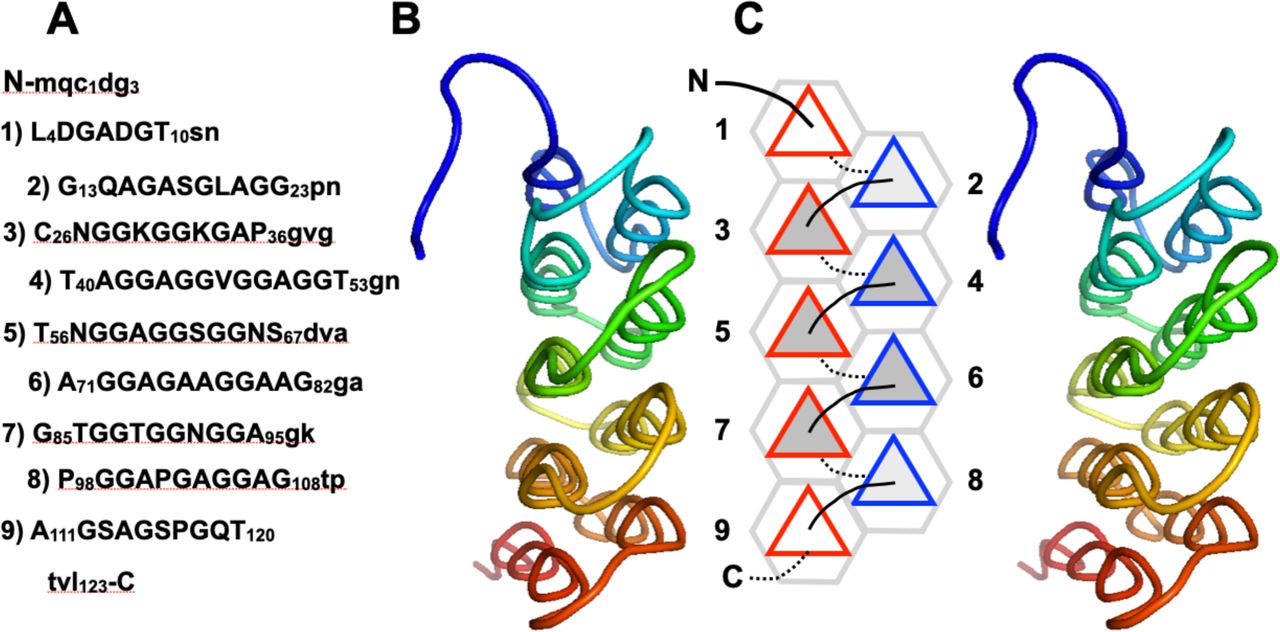
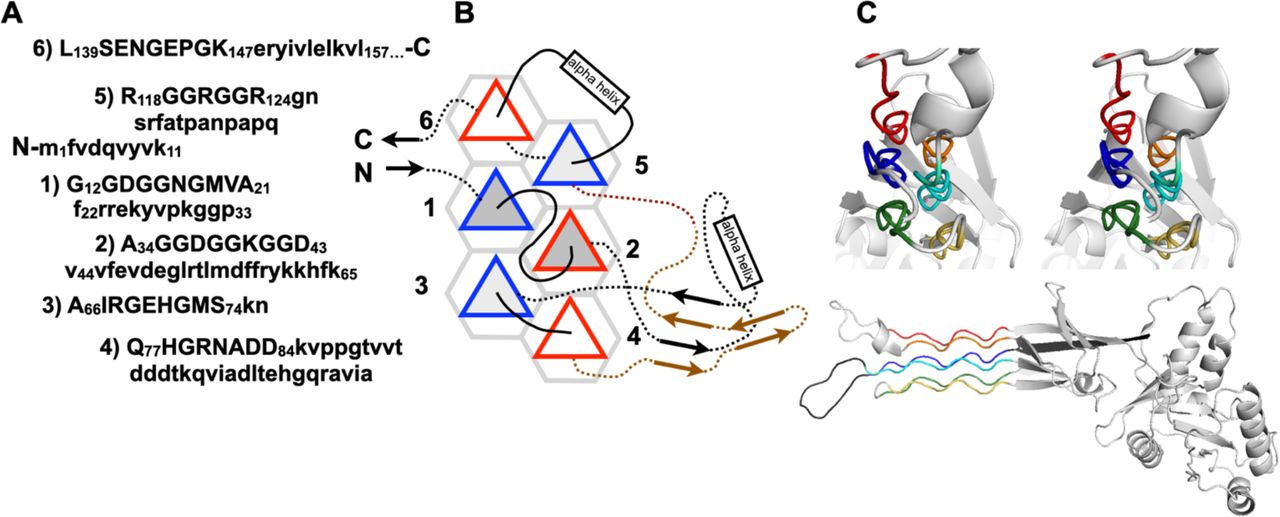
File:PPIIMoL Fig1.jpg|alt=Four panels showing polyproline motifs and packing diagrams.|'''Figure 1.''' Overview of polyproline motifs.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
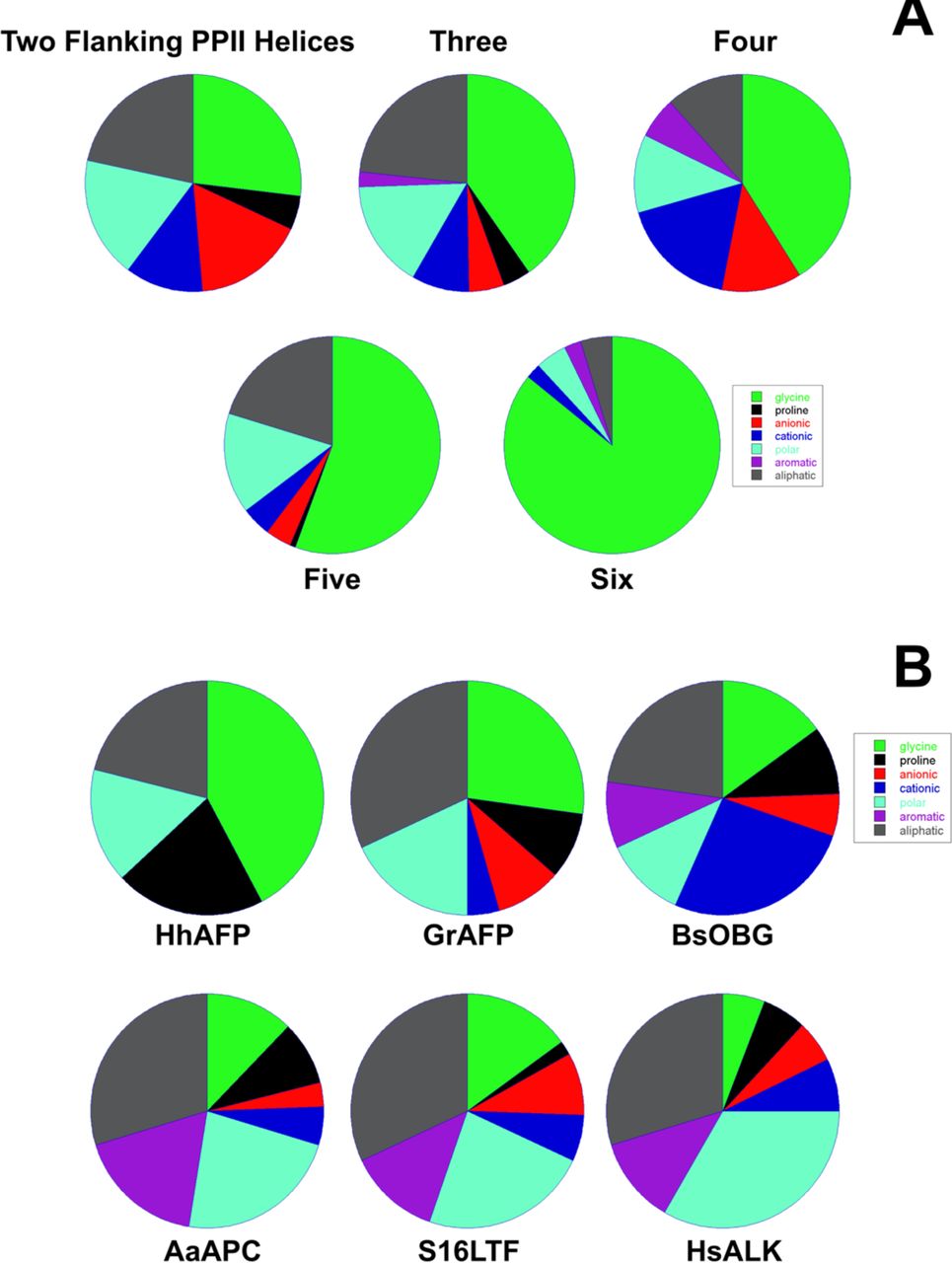
File:PPIIMoL | File:PPIIMoL Fig8.jpg|alt=Pie charts summarizing glycine content and flanking residues across PPII bundles.|'''Figure 8. Quantitative trends:'''<br>(A) Glycine content per PPII helix increases with number of neighbors.<br>(B) Flanking segments enriched in small polar/turn-forming residues; cationic residues often near C-termini.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | ||
</gallery> | </gallery> | ||
<!-- Filas siguientes: Fig. 2–7 --> | |||
<gallery widths=500 heights=340 perrow=2> <!-- añade mode=packed si tu wiki lo soporta --> | |||
File:PPIIMoL Fig2.jpg|alt=HhAFP with six PPII helices arranged in two antiparallel layers and disulfides.|'''Figure 2.''' Snow flea antifreeze protein (HhAFP): six PPII helices in two layers, stabilized by disulfides and predominantly antiparallel.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
File:PPIIMoL Fig3.jpg|alt=GrAFP nine-helix PPII bundle in two layers.|'''Figure 3.''' Granisotoma rainieri antifreeze protein (GrAFP): nine-helix PPII bundle arranged in two layers.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
File:PPIIMoL Fig4.jpg|alt=Obg GTPase domain with six PPII helices connected by variable segments.|'''Figure 4.''' Obg GTPase PPII domain: six PPII helices in two layers connected by variable segments.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
File:PPIIMoL Fig5.jpg|alt=Carboxylases forming compact bundles of short PPII helices.|'''Figure 5.''' Carboxylases: compact bundles of short PPII helices; one helix surrounded by six neighbors; bundle largely buried.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
File:PPIIMoL Fig6.jpg|alt=Bacteriophage S16 tail fiber tip with ten PPII helices and variable loops.|'''Figure 6.''' Bacteriophage S16 tail fiber tip (gp38): ten PPII helices; two fully glycine and fully surrounded; variable loops control host recognition.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
File:PPIIMoL Fig7.jpg|alt=Human ALK extracellular glycine-rich domain with fourteen PPII helices.|'''Figure 7.''' Human ALK extracellular glycine-rich domain: fourteen PPII helices; three nearly all-Gly; connectors range from short turns to longer elements.<br><small>Reproduced with permission from Segura Rodríguez & Laurents (2024).</small> | |||
</gallery> | |||
== GUI buttons == | == GUI buttons == | ||
| Line 89: | Line 107: | ||
* Too many contacts → Tighten cutoffs in Scan Cα–H···O=C. | * Too many contacts → Tighten cutoffs in Scan Cα–H···O=C. | ||
* CSV/PDB not written → Verify write permissions. | * CSV/PDB not written → Verify write permissions. | ||
* Emojis not visible → Cosmetic only. | * Emojis not visible → Cosmetic only. | ||
== Compatibility == | |||
* PyMOL: 2.x (Tkinter required for the GUI) | |||
* OS: Windows / Linux / macOS | |||
* Python: 3.x | |||
* Notes: requires complete backbone atoms to compute φ/ψ; add hydrogens to enable geometric screening. | |||
== Support == | |||
Found a bug or have a feature request? → [https://github.com/silviaenma/PPIIMoL/issues Open an issue on GitHub]. | |||
== How to cite == | == How to cite == | ||
| Line 98: | Line 125: | ||
'''Reference article''' | '''Reference article''' | ||
Segura Rodríguez, C. M., & Laurents, D. V. (2024). Architectonic principles of polyproline II helix bundle protein domains. ''Archives of Biochemistry and Biophysics, 741'', 109981. https://doi.org/10.1016/j.abb.2024.109981 | Segura Rodríguez, C. M., & Laurents, D. V. (2024). Architectonic principles of polyproline II helix bundle protein domains. ''Archives of Biochemistry and Biophysics, 741'', 109981. [https://doi.org/10.1016/j.abb.2024.109981 DOI] | ||
== Repository == | == Repository == | ||
[https://github.com/silviaenma/PPIIMoL PPIIMoL on GitHub] | |||
== License == | == License == | ||
PPIIMoL is released under the GNU GPLv3. | PPIIMoL is released under the [https://www.gnu.org/licenses/gpl-3.0.en.html GNU GPLv3]. | ||
[[Category:Plugins]] | [[Category:Plugins]] | ||
[[Category:Structure Analysis]] | |||
Latest revision as of 02:16, 19 August 2025
PPIIMoL
PPIIMoL is a Python module for PyMOL that automates the detection of polyproline II (PPII) helices in proteins. It identifies PPII-like φ/ψ angle patterns, screens for plausible non-canonical Cα–H···O=C contacts, and provides one-click visualization and export.
This tool was developed as part of a Bachelor's Thesis in Computer Engineering in collaboration with the Protein Structure, Dynamics and Interactions by NMR Group at the Instituto de Química-Física “Blas Cabrera” (IQF-CSIC). The module’s design and validation take as a primary reference the data and architectonic principles reported by Segura Rodríguez & Laurents (2024) (see How to cite).
Scientific background
Polyproline II (PPII) helices are extended, left-handed motifs (≈3 residues/turn) typically enriched in glycine- and proline-rich domains. Although common in several glycine-rich bundles, they are often unannotated in PDB files. PPIIMoL automates their detection directly in PyMOL to improve speed and reproducibility.
Features
- 🔍 Automatic detection of PPII segments via phi/psi angle analysis.
- 🧬 Identification of Cα-H···O=C interactions relevant to structural stability.
- 📊 CSV export of detected segments and interactions.
- 🎨 Direct visualization in PyMOL with customizable color codes.
- 🖱️ Simple Tkinter-based GUI — no commands required; all actions are accessible via buttons.
Requirements
- PyMOL 2.x or newer.
- Python with Tkinter enabled (for the GUI).
Installation
Option A — Single-file download (simplest)
- Download `PPIIMoL.py` from the repository (see Repository below).
- In PyMOL:
run /full/path/to/PPIIMoL.py
Option B — Clone the repository
git clone https://github.com/silviaenma/PPIIMoL.git
Then in PyMOL:
run PPIIMoL/PPIIMoL.py
Optional: install as a plugin In PyMOL: Plugin → Plugin Manager → Install New Plugin → select `PPIIMoL.py` (or the whole folder) → restart PyMOL.
Usage (GUI)
Once loaded, PPIIMoL opens a Tkinter window with buttons to:
- Load PDB (or use an already-loaded object)
- Detect PPII (scan φ/ψ windows and list segments)
- Scan Cα–H···O=C (optional geometric screening)
- Style / Colors (apply the chosen palette)
- Export (CSV reports; optional per-segment PDBs)
Results are written to a date-stamped folder; selections/objects are created in the PyMOL session and colored according to the chosen scheme.
GUI snapshot
Examples
Example (command line, optional)
# Load the module run /full/path/to/PPIIMoL.py # or run PPIIMoL/PPIIMoL.py # Load a structure and trigger detection fetch 3bog, async=0 ppii_detect()
Reference figures
Below are reference figures illustrating PPII bundle organization and residue patterns, reproduced with permission from Segura Rodríguez & Laurents (2024).
GUI buttons
- Load PDB: Opens a file dialog (.pdb/.cif).
- Prepare structure: Optional cleanup (remove solvent/ligands, add hydrogens).
- Detect PPII: Scans backbone torsion angles (φ/ψ), creates selections (ppii_1, ppii_2, …) and colors them.
- Scan Cα–H···O=C: Searches plausible non-canonical contacts; cutoffs configurable.
- Style / Colors: Applies the selected color scheme.
- Export: Writes CSV reports (angles, contacts, detected segments).
Troubleshooting
- GUI does not appear → Ensure PyMOL build includes Tkinter.
- No segments detected → Check backbone completeness; relax φ/ψ windows.
- Too many contacts → Tighten cutoffs in Scan Cα–H···O=C.
- CSV/PDB not written → Verify write permissions.
- Emojis not visible → Cosmetic only.
Compatibility
- PyMOL: 2.x (Tkinter required for the GUI)
- OS: Windows / Linux / macOS
- Python: 3.x
- Notes: requires complete backbone atoms to compute φ/ψ; add hydrogens to enable geometric screening.
Support
Found a bug or have a feature request? → Open an issue on GitHub.
How to cite
If PPIIMoL is useful in your work, please cite both the software and the reference article:
Software Rodríguez Fernández, S. E. (2025). PPIIMoL (version 1.1) [Computer software]. GitHub. https://github.com/silviaenma/PPIIMoL
Reference article Segura Rodríguez, C. M., & Laurents, D. V. (2024). Architectonic principles of polyproline II helix bundle protein domains. Archives of Biochemistry and Biophysics, 741, 109981. DOI
Repository
License
PPIIMoL is released under the GNU GPLv3.