PLoS: Difference between revisions
No edit summary |
|||
| Line 12: | Line 12: | ||
Illustration of the overall fold of carbonic anhydrase, CsoS3, from Halothiobacillus neapolitanus.(PDB ID 2G13). The three domains that compose this enzyme are distinguished by coloring each domain separately (blue, yellow, red). The orientation was chosen to feature the location of the active site (outlined by side chains and zinc ion), and to show the two-fold symmetry relationship between the active domain (yellow) and homologous but defunct domain (red). SSEs are labeled directly on the individual SSEs. Domain labels are colored to correspond to the domains they are labeling. Labels in the active site are given an “outer glow” to make them legible in a region of the figure that is dense in detail. Depth is conveyed by use of fog, veiling less important structural features in the back of the enzyme. | Illustration of the overall fold of carbonic anhydrase, CsoS3, from Halothiobacillus neapolitanus.(PDB ID 2G13). The three domains that compose this enzyme are distinguished by coloring each domain separately (blue, yellow, red). The orientation was chosen to feature the location of the active site (outlined by side chains and zinc ion), and to show the two-fold symmetry relationship between the active domain (yellow) and homologous but defunct domain (red). SSEs are labeled directly on the individual SSEs. Domain labels are colored to correspond to the domains they are labeling. Labels in the active site are given an “outer glow” to make them legible in a region of the figure that is dense in detail. Depth is conveyed by use of fog, veiling less important structural features in the back of the enzyme. | ||
< | <source lang="python"> | ||
#BEGIN PYMOL SCRIPT for Overall Fold -left half of stereo image | #BEGIN PYMOL SCRIPT for Overall Fold -left half of stereo image | ||
| Line 101: | Line 101: | ||
# Show sticks for bonds | # Show sticks for bonds | ||
show sticks, (resid 173 or resid 175 or resid 177 or resid 242 or resid 253) and not (name n or name c or name o) | show sticks, (resid 173 or resid 175 or resid 177 or resid 242 or resid 253) and not (name n or name c or name o) | ||
set_view (\ | set_view (\ | ||
| Line 115: | Line 114: | ||
ray | ray | ||
png csos3-left.png | png csos3-left.png | ||
</ | </source> | ||
===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 2:''' Ligand-binding sites (≥ novice/intermediate)</div>=== | ===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 2:''' Ligand-binding sites (≥ novice/intermediate)</div>=== | ||
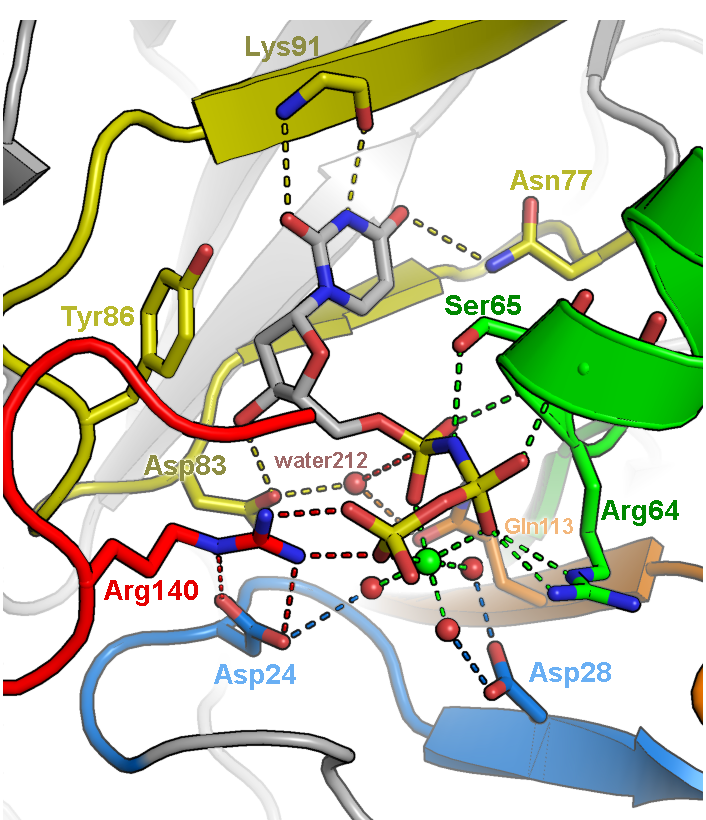
[[File:Dutpase active02-labeled.png|200px|thumb|left|Active Site Figure]]Illustration of the active site of dUTPase from Mycobacterium tuberculosis (PDB ID 1SIX). The orientation of the active site was chosen to feature the geometry of the chemical reaction catalyzed by this enzyme, specifically the in-line nucleophilic attack of water 212 on the alpha-phosphate of dUTP. Fine tuning of the orientation was made to eliminate overlap of side chains and to make all hydrogen bonds (dashed lines) visible. Only side chains directly involved in catalysis are depicted. Carbons are colored according to five conserved motifs of the dUTPase family. Non-conserved residues are given an undistracting gray color and are veiled in fog. Labels were colored to correspond to the side chains they are labeling. | [[File:Dutpase active02-labeled.png|200px|thumb|left|Active Site Figure]]Illustration of the active site of dUTPase from Mycobacterium tuberculosis (PDB ID 1SIX). The orientation of the active site was chosen to feature the geometry of the chemical reaction catalyzed by this enzyme, specifically the in-line nucleophilic attack of water 212 on the alpha-phosphate of dUTP. Fine tuning of the orientation was made to eliminate overlap of side chains and to make all hydrogen bonds (dashed lines) visible. Only side chains directly involved in catalysis are depicted. Carbons are colored according to five conserved motifs of the dUTPase family. Non-conserved residues are given an undistracting gray color and are veiled in fog. Labels were colored to correspond to the side chains they are labeling. | ||
< | <source lang="python"> | ||
#BEGIN PYMOL SCRIPT for Active Site | #BEGIN PYMOL SCRIPT for Active Site | ||
| Line 244: | Line 243: | ||
# ray 1500,1500 | # ray 1500,1500 | ||
viewport 700,800 | viewport 700,800 | ||
</ | </source> | ||
===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 3:''' Structure alignment (≥ novice/intermediate)</div>=== | ===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 3:''' Structure alignment (≥ novice/intermediate)</div>=== | ||
| Line 251: | Line 250: | ||
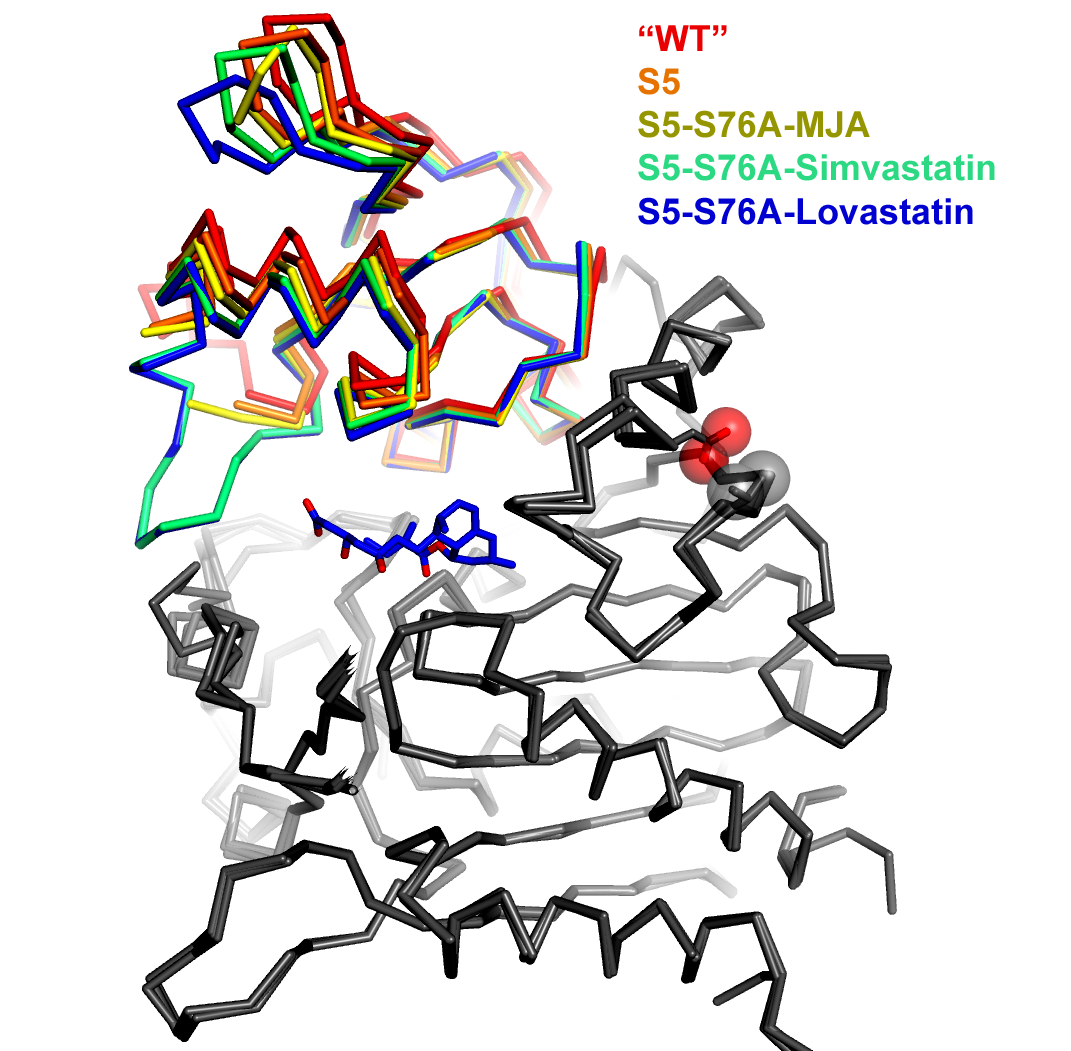
An overlay of five simvastatin synthetase crystal structures illustrating degrees of hinge closing imparted by ligand binding (PDB IDs 3HLB, 3HLC, 3HLE, 3HLF, and 3HLG). Hinge motion in this two-domain enzyme is highlighted by superimposing only atoms in one of the domains (depicted in gray in this figure). The range of motion is highlighted by the rainbow colors assigned to the upper domain. The orientation of the molecule is chosen to make the range of motion evident (hinge axis is normal to the plane of the page). Each of the structures is labeled explicitly in the figure, rather than burying the information in the figure legend. Color coding the labels makes it easier to comprehend how each ligand affects the hinge motion. The structures are represented as a alpha-carbon trace rather than a cartoon ribbon because the motion is relatively small and the alpha carbon trace allows a more exact representation of the position of the atoms. | An overlay of five simvastatin synthetase crystal structures illustrating degrees of hinge closing imparted by ligand binding (PDB IDs 3HLB, 3HLC, 3HLE, 3HLF, and 3HLG). Hinge motion in this two-domain enzyme is highlighted by superimposing only atoms in one of the domains (depicted in gray in this figure). The range of motion is highlighted by the rainbow colors assigned to the upper domain. The orientation of the molecule is chosen to make the range of motion evident (hinge axis is normal to the plane of the page). Each of the structures is labeled explicitly in the figure, rather than burying the information in the figure legend. Color coding the labels makes it easier to comprehend how each ligand affects the hinge motion. The structures are represented as a alpha-carbon trace rather than a cartoon ribbon because the motion is relatively small and the alpha carbon trace allows a more exact representation of the position of the atoms. | ||
< | <source lang="python"> | ||
#BEGIN PYMOL SCRIPT for Overlay of Aligned Molecules | #BEGIN PYMOL SCRIPT for Overlay of Aligned Molecules | ||
| Line 301: | Line 300: | ||
171.387252808, 203.264083862, 0.000000000 ) | 171.387252808, 203.264083862, 0.000000000 ) | ||
~ | ~ | ||
</ | </source> | ||
===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 4:''' Volumetric data (≥ intermediate)</div>=== | ===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 4:''' Volumetric data (≥ intermediate)</div>=== | ||
| Line 308: | Line 307: | ||
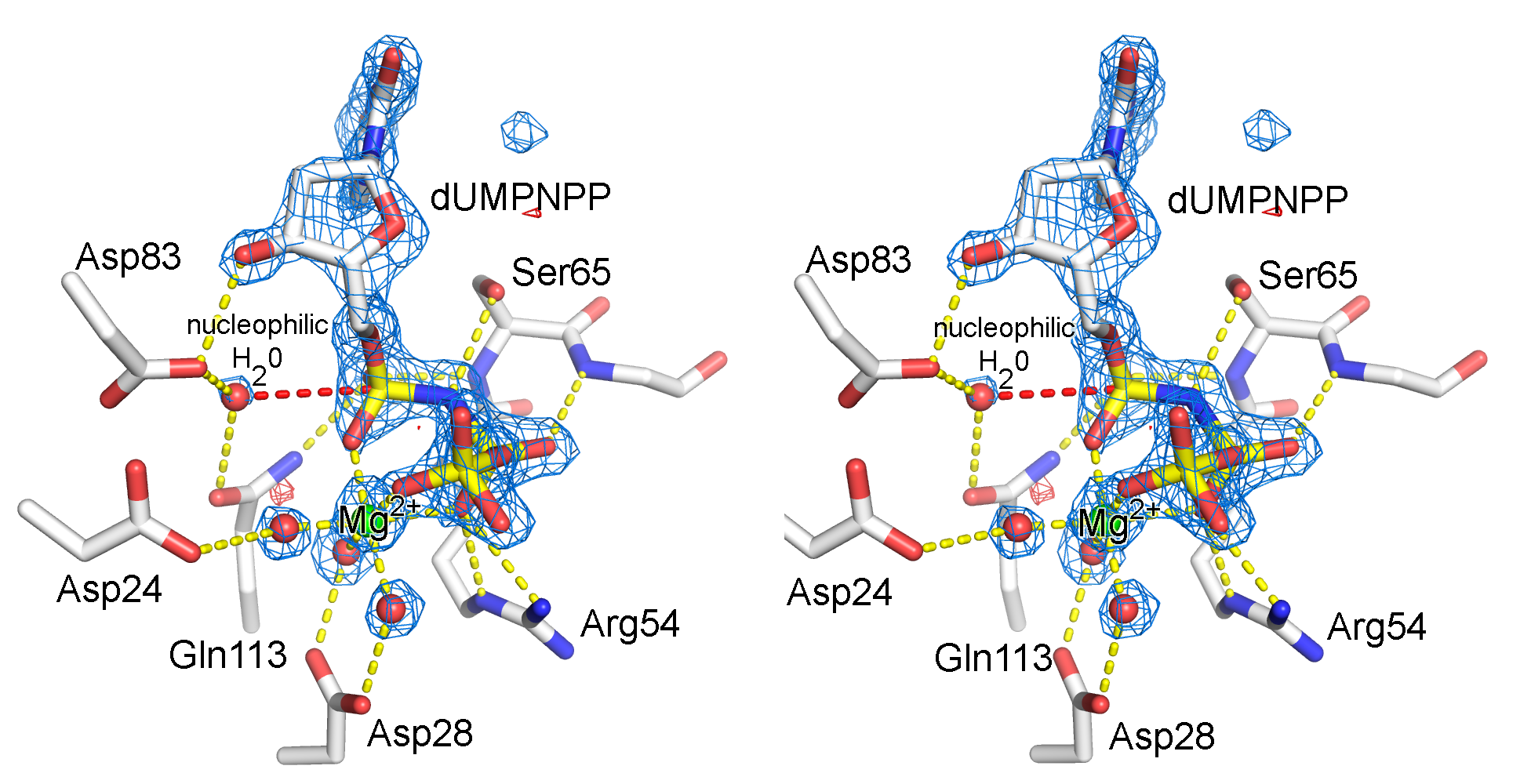
Illustration of an electron density map (omit map) from the enzyme, dUTPase from Mycobacterium tuberulosis (PDB ID 1SIX). | Illustration of an electron density map (omit map) from the enzyme, dUTPase from Mycobacterium tuberulosis (PDB ID 1SIX). | ||
< | <source lang="python"> | ||
#BEGIN PYMOL SCRIPT Electron Density Map -Left half of stereo image | #BEGIN PYMOL SCRIPT Electron Density Map -Left half of stereo image | ||
| Line 381: | Line 380: | ||
hide labels | hide labels | ||
set_view (\ | set_view (\ | ||
| Line 397: | Line 394: | ||
#ray | #ray | ||
#png density-left.png | #png density-left.png | ||
</ | </source> | ||
===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 5:''' Interfaces (≥ intermediate)</div>=== | ===<div style="background-color:#eeecde; margin-top:0px;border-bottom: 1px dotted #999999; padding-top:3px;font-weight:normal;">'''Case 5:''' Interfaces (≥ intermediate)</div>=== | ||
Revision as of 21:23, 30 December 2009
Biomolecular Graphics: In Principle and in Practice
Overview, general notes
Blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah blah
Figures
This section illustrates the figure-creation process, from initial conception → final multi-panel figure ready for publication. Organized as various "case studies" that are representative of the tasks typically faced by structural or computational biologists, this section provides several step-by-step examples. These examples are entirely self-contained, and the materials include everything from (i) raw, starting data (e.g., PDB files) to (ii) actual, working PyMOL or Python scripts and (iii) final output image files.
Case 1: Overall fold / domain level (≥ novice/intermediate)
Illustration of the overall fold of carbonic anhydrase, CsoS3, from Halothiobacillus neapolitanus.(PDB ID 2G13). The three domains that compose this enzyme are distinguished by coloring each domain separately (blue, yellow, red). The orientation was chosen to feature the location of the active site (outlined by side chains and zinc ion), and to show the two-fold symmetry relationship between the active domain (yellow) and homologous but defunct domain (red). SSEs are labeled directly on the individual SSEs. Domain labels are colored to correspond to the domains they are labeling. Labels in the active site are given an “outer glow” to make them legible in a region of the figure that is dense in detail. Depth is conveyed by use of fog, veiling less important structural features in the back of the enzyme.
#BEGIN PYMOL SCRIPT for Overall Fold -left half of stereo image
# antialias =1 smooths jagged edges, 0 turns it off
set antialias = 1
# Larger values of ambient make the image brighter
set ambient=0.3
# Larger values of direct eliminates shadows
set direct=1.0
set ribbon_radius =0.2
# cartoon_highlight_color will give a separate color to edges of secondary structural elements.
set cartoon_highlight_color =grey50
# ray_trace_mode=1 makes a black outline around the secondary structural elements
set ray_trace_mode=1
# stick_radius -adjust thickness of atomic bonds
set stick_radius = 0.2
# mesh_radius -to adjust thickness of electron density contours
set mesh_radius = 0.02
# bg_color --set the background color
bg_color white
# load pdb file and give it an object name
load csos3_18o_nobreak.pdb, csos3
# hide nonbonded atoms (i.e. waters)
hide nonbonded
# show cartoon ribbons
show cartoon
# Hide the default line representation of atomic bonds
hide lines
# Use standard helix, strand, and loop representations
# other possibilities: cartoon loop, cartoon rect,
# cartoon oval, and cartoon tube
set cartoon_tube_radius, 0.2
# If you dont have secondary structure assignments
# in the PDB header then get secondary structure assignments
# from dssp http://swift.cmbi.kun.nl/gv/dssp/
# Then convert assignments into a PDB header
# http://structure.usc.edu/dssp2pdb/
# If you want to define secondary structure manually,
# Use the following syntax 'S'=strand 'H'=helix
# alter B/753:758/, ss='S'
# Make fancy helices with ridge on the edges like
# molscript does
# 1 is on. 0 is off.
set cartoon_fancy_helices=1
set cartoon_cylindrical_helices=0
# Make the strands flat=1 or pass through CA positions=0
# Set to 0 when showing side chains from a strand
set cartoon_flat_sheets = 1.0
# Draw the loops smooth=1 or pass through CA positions=0
# Set to 0 when showing side chains from a loop
set cartoon_smooth_loops = 0
# Set the color of the residues
# to find the names of the colors available
# click on the rainbow colored square in the
# upper right corner of the graphics window
# Here I make some customized colors as mixtures of red,green,blue
set_color maarine= [0.3, 0.8, 1.0]
set_color graay=[0.8,0.8,0.8]
set_color greeen=[0.0,0.5,0.0]
color gray50, elem C
color greeen, elem ZN
color blue, resid 38:147 and name ca
color yellow, resid 148:397 and name ca
color red, resid 398:514 and name ca
# Show spheres for chloride ions
show spheres, elem ZN
# Show sticks for bonds
show sticks, (resid 173 or resid 175 or resid 177 or resid 242 or resid 253) and not (name n or name c or name o)
set_view (\
0.091340274, -0.606698275, 0.789650559,\
-0.991202235, -0.131515890, 0.013612081,\
0.095602803, -0.783963323, -0.613382638,\
0.001799395, 0.001679182, -246.492980957,\
12.976243019, 41.245639801, 62.928291321,\
187.538497925, 249.492980957, 0.000000000 )
turn y, 3.5
viewport 1200,1500
ray
png csos3-left.png
Case 2: Ligand-binding sites (≥ novice/intermediate)
Illustration of the active site of dUTPase from Mycobacterium tuberculosis (PDB ID 1SIX). The orientation of the active site was chosen to feature the geometry of the chemical reaction catalyzed by this enzyme, specifically the in-line nucleophilic attack of water 212 on the alpha-phosphate of dUTP. Fine tuning of the orientation was made to eliminate overlap of side chains and to make all hydrogen bonds (dashed lines) visible. Only side chains directly involved in catalysis are depicted. Carbons are colored according to five conserved motifs of the dUTPase family. Non-conserved residues are given an undistracting gray color and are veiled in fog. Labels were colored to correspond to the side chains they are labeling.
#BEGIN PYMOL SCRIPT for Active Site
set mesh_radius = 0.01
set antialias = 1
set stick_radius = 0.22
set dash_radius=0.07
set sphere_scale= 0.22
set ribbon_radius =0.1
set direct =0.0
set cartoon_fancy_helices=1
bg_color white
set gamma=1.5
util.ray_shadows('none')
set ray_trace_mode=1
# load pdb and map file
load dumpnpp_10t.pdb, mtb
cartoon automatic
show cartoon
hide nonbonded
hide lines
color white, (chain A or chain B or chain C) and (n;ca)
color yellow, (resid 171) or (n;PA) or (n;PB) or (n;PG)
color white, (elem C)
#magnesium
show sphere, (resid 171)
color green, (resid 171)
#waters
show sphere, (resid 173:175 or resid 212)
show sticks, (chain C and resid 170)
color marine, (resid 22:32 and elem C) and chain C
color green, (resid 57:76 and elem C) and chain C
color yellow, (resid 77:93 and elem C) and chain A
color orange, (resid 102:115 and elem C) and chain C
color red, (resid 134:145 and elem C ) and chain B
show sticks, (chain A and resid 83 and not n;n,c,o )
show sticks, (chain A and resid 86 and not n;n,c,o )
show sticks, (chain A and resid 77 and not n;n,c,o )
show sticks, (chain C and resid 24 and not n;n,c,o)
show sticks, (chain C and resid 28 and not n;n,c,o)
show sticks, (chain B and resid 140 and not n;n,c,o)
show sticks, (chain C and resid 64 and not n;n,c,o)
show sticks, (chain C and resid 113 and not n;n,c,o)
show sticks, (chain C and resid 64:66 )
hide sticks, (chain C and resid 64 and n;n,c,o)
show sticks, (chain A and resid 91 and n;n,c,o,ca)
distance (resid 171 and chain C), (resid 173 and chain C)
color green, dist01
distance (resid 171 and chain C), (resid 174 and chain C)
color green , dist02
distance (resid 171 and chain C), (resid 175 and chain C)
color green , dist03
distance (resid 171 and chain C), (resid 170 and chain C and n;O1A)
color green , dist04
distance (resid 171 and chain C), (resid 170 and chain C and n;O1B)
color green , dist05
distance (resid 171 and chain C), (resid 170 and chain C and n;O1G)
color green , dist06
distance (resid 173 and chain C), (resid 28 and chain C and n;OD2)
color marine, dist07
distance (resid 174 and chain C), (resid 28 and chain C and n;OD1)
color marine, dist08
distance (resid 175 and chain C), (resid 24 and chain C and n;OD2)
color marine, dist09
distance (resid 212 and chain A), (resid 170 and chain C and n;PA)
color red, dist10
distance (resid 212 and chain A), (resid 113 and chain C and n;OE1)
color orange, dist11
distance (resid 212 and chain A), (resid 83 and chain A and n;OD2)
color yellow, dist12
distance (resid 113 and chain C and n;ne2), (resid 170 and chain C and n;O3A)
color orange, dist13
distance (resid 65 and chain C and n;N), (resid 170 and chain C and n;O3A)
color green, dist14
distance (resid 65 and chain C and n;OG), (resid 170 and chain C and n;N2A)
color green, dist15
distance (resid 66 and chain C and n;N), (resid 170 and chain C and n;O3B)
color green, dist16
distance (resid 64 and chain C and n;NH2), (resid 170 and chain C and n;O1B)
color green, dist17
distance (resid 64 and chain C and n;NE), (resid 170 and chain C and n;O1B)
color green, dist18
distance (resid 83 and chain A and n;OD2), (resid 170 and chain C and n;O3')
color yellow, dist19
distance (resid 140 and chain B and n;NH1), (resid 170 and chain C and n;O2G)
color red, dist20
distance (resid 140 and chain B and n;NH2), (resid 170 and chain C and n;O1G)
color red, dist21
distance (resid 140 and chain B and n;NE), (resid 24 and chain C and n;OD1)
color red, dist22
distance (resid 140 and chain B and n;NH2), (resid 24 and chain C and n;OD2)
color red, dist23
distance (resid 170 and chain C and n;O4), (resid 77 and chain A and n;ND2)
color yellow, dist24
distance (resid 170 and chain C and n;N3), (resid 91 and chain A and n;O)
color yellow, dist24
distance (resid 170 and chain C and n;O2), (resid 91 and chain A and n;N)
color yellow, dist24
hide labels
set cartoon_flat_sheets = 1.0
set cartoon_smooth_loops = 0
set_view (\
0.045321193, 0.677332938, -0.734195530,\
0.631984174, -0.588606596, -0.504004478,\
-0.773574233, -0.441189915, -0.454773396,\
0.000917640, 0.000133177, -65.177696228,\
14.812702179, 8.131608009, 17.861175537,\
54.978454590, 79.653022766, 0.000000000 )
# ray 1500,1500
viewport 700,800
Case 3: Structure alignment (≥ novice/intermediate)
An overlay of five simvastatin synthetase crystal structures illustrating degrees of hinge closing imparted by ligand binding (PDB IDs 3HLB, 3HLC, 3HLE, 3HLF, and 3HLG). Hinge motion in this two-domain enzyme is highlighted by superimposing only atoms in one of the domains (depicted in gray in this figure). The range of motion is highlighted by the rainbow colors assigned to the upper domain. The orientation of the molecule is chosen to make the range of motion evident (hinge axis is normal to the plane of the page). Each of the structures is labeled explicitly in the figure, rather than burying the information in the figure legend. Color coding the labels makes it easier to comprehend how each ligand affects the hinge motion. The structures are represented as a alpha-carbon trace rather than a cartoon ribbon because the motion is relatively small and the alpha carbon trace allows a more exact representation of the position of the atoms.
#BEGIN PYMOL SCRIPT for Overlay of Aligned Molecules
#Load the individual, superimposed molecules
load lovd_c2_refmac10-b_lsq.pdb, G0-Se
load lovd-gx27_refmac11_lsq.pdb, G5
load lovd_gx27-s76a-mja_aps-refmac3_lsq.pdb, G5-S76-MJA
load lovd-gx27-s76a-sim-aps_refmac6_lsq.pdb, G5-S76-Sim
load lovd-gx27-s76a-lov-aps_refmac6.pdb, G5-S76-Lov
hide everything
show ribbon
set ray_trace_mode=0
color red, G0-Se
color orange, G5
color yellow, G5-S76-MJA
color limegreen, G5-S76-Sim
color blue, G5-S76-Lov
color gray, resid 1:92 or resid 204:413
set ribbon_radius=0.3
show sticks, resid 134 and not (name C or name N or name O)
show sticks, resid 86 and not (name C or name N or name O)
show sticks, resn 803
show sticks, G5-MJA and resid 501
show sticks, resid 320 and not (name C or name N or name O) and ( G5-S76-Sim or G5-S76-Lov)
show sticks, resid 334 and not (name C or name N or name O) and ( G5-S76-Sim or G5-S76-Lov)
show spheres, resid 320 and (name od1 or name od2) and G5-S76-Sim
show spheres, resid 334 and (name cg1 or name cg2) and G5-S76-Sim
color red, elem o
set sphere_transparency=0.5
set ray_shadow=off
bg_color white
viewport 1071,1051
set_view (\
0.836338997, 0.441327691, -0.325210959,\
0.390257686, -0.062672906, 0.918569088,\
0.385009199, -0.895153403, -0.224648848,\
-0.000003189, 0.000000304, -189.126144409,\
1.563406944, 17.993612289, -24.687477112,\
171.387252808, 203.264083862, 0.000000000 )
~
Case 4: Volumetric data (≥ intermediate)
Illustration of an electron density map (omit map) from the enzyme, dUTPase from Mycobacterium tuberulosis (PDB ID 1SIX).
#BEGIN PYMOL SCRIPT Electron Density Map -Left half of stereo image
# general settings
set mesh_radius = 0.015
set antialias = 1
set stick_radius = 0.22
set sphere_scale= 0.22
set ribbon_radius =0.1
# higher values of direct will lighten the image
set direct =0.5
# make the dash lines visible by increasing the thicknes of the dash_radius
set dash_radius = 0.1
#turn of shadows because they complicate the image
util.ray_shadows('none')
bg_color white
# load pdb and map file
load dumpnpp_10t.pdb, mtb
load omit_twin_out.xplor, 1fofc
# show e-density nearby
# show positive contours of the map 4.2 sigma or higher within 18 angstroms of residue 170 of chain C
isomesh pos, 1fofc, 4.2, (resid 170 and chain C ), 18.0
color marine, pos
# show negative contours of the map -4.2 sigma or lower within 18 angstroms of residue 170 of chain C
isomesh neg, 1fofc, -4.2, (resid 170 and chain C ), 18.0
color red, neg
cartoon automatic
hide nonbonded
hide lines
color white, (chain A or chain B or chain C) and (n;ca)
color yellow, (resid 171) or (n;PA) or (n;PB) or (n;PG)
color white, (elem C)
#magnesium
show sphere, (resid 171)
color green, (resid 171)
#waters
show sphere, (resid 173:175 or resid 212)
show sticks, (chain C and resid 170)
show sticks, (chain A and resid 83 and not n;n,c,o )
show sticks, (chain C and resid 24 and not n;n,c,o)
show sticks, (chain C and resid 28 and not n;n,c,o)
show sticks, (chain C and resid 64 and not n;n,c,o)
show sticks, (chain C and resid 113 and not n;n,c,o)
show sticks, (chain C and resid 64:66 )
distance (resid 171 and chain C), (resid 173 and chain C)
distance (resid 171 and chain C), (resid 174 and chain C)
distance (resid 171 and chain C), (resid 175 and chain C)
distance (resid 171 and chain C), (resid 170 and chain C and n;O1A)
distance (resid 171 and chain C), (resid 170 and chain C and n;O1B)
distance (resid 171 and chain C), (resid 170 and chain C and n;O1G)
distance (resid 173 and chain C), (resid 28 and chain C and n;OD2)
distance (resid 174 and chain C), (resid 28 and chain C and n;OD1)
distance (resid 175 and chain C), (resid 24 and chain C and n;OD2)
distance (resid 212 and chain A), (resid 170 and chain C and n;PA)
distance (resid 212 and chain A), (resid 113 and chain C and n;OE1)
distance (resid 212 and chain A), (resid 83 and chain A and n;OD2)
distance (resid 113 and chain C and n;ne2), (resid 170 and chain C and n;O3A)
distance (resid 65 and chain C and n;N), (resid 170 and chain C and n;O3A)
distance (resid 65 and chain C and n;OG), (resid 170 and chain C and n;N2A)
distance (resid 66 and chain C and n;N), (resid 170 and chain C and n;O3B)
distance (resid 64 and chain C and n;NH2), (resid 170 and chain C and n;O1B)
distance (resid 64 and chain C and n;NE), (resid 170 and chain C and n;O1B)
distance (resid 83 and chain A and n;OD2), (resid 170 and chain C and n;O3')
color yellow, dist*
color red, dist10
hide labels
set_view (\
-0.350610733, 0.599414885, -0.719504118,\
-0.160659447, -0.795388758, -0.584337831,\
-0.922586679, -0.089291766, 0.375185639,\
0.002084959, 0.000028796, -51.327602386,\
14.307765961, 9.915379524, 17.268175125,\
42.648197174, 61.923065186, 0.000000000 )
turn y, 3.5
viewport 1000, 1000
#ray
#png density-left.png
Case 5: Interfaces (≥ intermediate)
Illustration of an intermolecular interface from the PE-PPE protein complex from Mycobacterium tuberulosis (PDB ID 2G38). The left panel illustrates the complex between PE and PPE proteins as a cartoon ribbon. The two proteins are colored separately to make the interface evident. Hydrophobic side chains involved in the interface are labeled. The right panel illustrates the complementary surfaces of the interface by splitting the complex apart like a clamshell. The smaller protein is the PE protein, the larger protein is the PPE protein. Warm colors indicate strong hydrophobicity; cool colors depict weak hydrophobicity. The left and right panels are depicted on the same scale so the viewer can see how residues labeled on the left correspond to red patches in the surface on the right.
Case 6: Higher-order structures (≥ intermediate/advanced)
Case 7: Animations (≥ intermediate/advanced)
See the Animations section below.
Case 8: Best-fit planes (≥ advanced)
Animations
A classification scheme
MsVs
These are just 'ordinary', static figures, as illustrated by the case studied above.
MsVd
MdVs
MdVd
Useful links
A compilation of links and other online resources that were helpful in creating these examples. Please edit as you see fit.