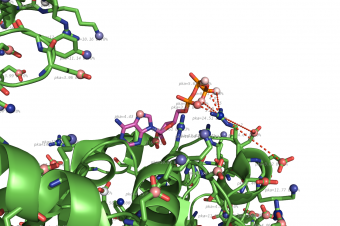
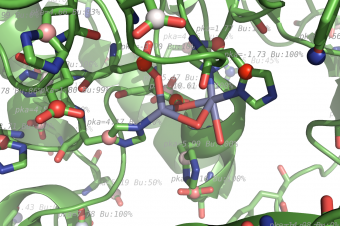
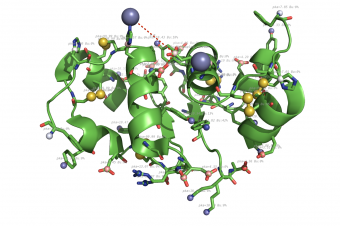
Propka
Author and Acknowledgement
This pymol script is made by Troels Emtekær Linnet
propka.py contact and relies on the result from the propka server
The PROPKA method is developed by the Jensen Research Group , Department of Chemistry, University of Copenhagen.
Introduction
This script can fetch the pka values for a protein from the propka server. The "propka" function downloads the results and processes them.
It also automatically writes a pymol command file and let pymol execute it. This command file make pka atoms, rename them, label them and color them according to the pka value.
If you put the mechanize folder and the propka.py script somewhere in your pymol search path, then getting the pka values is made super easy. By the way, did you know, that you don't have to prepare the .pdb file by adding/removing hydrogens? The propka server uses its own internal hydrogen placement algorithm.
import propka
fetch 4ins, async=0
propka
If there is no web connection, it is possible to process a result file from a previous run or from a downloaded propka webpage result. This can be a handsome feature in a teaching/seminar situation, since it speeds up the pymol result or that an available web connection can be doubtful. Just point to the .pka file: Remember the dot "." which means "current directory".
import propka
load 4ins.pdb
propka pkafile=./Results_propka/4ins"LOGTIME".pka, resi=18.25-30, resn=cys
The last possibility, is just to ask for the pka values of a recognized PDB id. This is done with the "getpropka" function.
import propka
getpropka source=ID, PDBID=4ake, logtime=_, showresult=yes
Dependency of python module: mechanize
The script needs mechanize to run.
- On windows, it is not easy to make additional modules available for pymol. So put in into your working folder.
- The easy manual way:
- Go to: http://wwwsearch.sourceforge.net/mechanize/download.html
- Download mechanize-0.2.5.zip. http://pypi.python.org/packages/source/m/mechanize/mechanize-0.2.5.zip
- Extract to .\mechanize-0.2.5 then move the in-side folder "mechanize" to your folder with propka.py. The rest of .\mechanize-0.2.5 you don't need.
- You can also see other places where you could put the "mechanize" folder. Write this in pymol to see the paths where pymol is searching for "mechanize"
- import sys; print(sys.path)
Examples
### Point to your directory with the script.
#cd /homes/linnet/Documents/Speciale/5NT-project/Mutant-construct/predict_reactivity/propka
cd C:/Users/tlinnet/Documents/My Dropbox/Speciale/5NT-project/Mutant-construct/predict_reactivity/propka
run ./propka.py OR
import propka
fetch 4ins, async=0
propka OR
propka 4ins OR
propka 4ins, resi=19.20, resn=ASP.TYR, logtime=_, verbose=yes
import propka
fetch 1hp1, async=0
propka molecule=1hp1, chain=A, resi=305-308.513, resn=CYS, logtime=_
import propka
getpropka source=ID, PDBID=4ins, logtime=_, server_wait=3.0, verbose=yes, showresult=yes
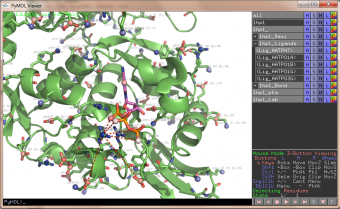
pka atoms are created and renamed for their pka value. That makes it easy to "click" the atom in pymol and instantly see the pka value.
The atoms b value are also altered to the pka value, and the atoms are then spectrum colored from pka=0-14.
The pka value of 99.9 represent a di-sulphide bond, and is colored gold and the sphere size is set a little bigger.
If one wants to see the specified result, the logfile ./Results_propka/_Results.log saves the link to the propka server. Here one can see in an interactive Jmol appp, the interactions to the pka residues.
Example Pymol Script
### Point to your directory with your pdb file and where to save the results
#cd /homes/linnet/Documents/Speciale/5NT-project/Mutant-construct/predict_reactivity/propka
cd C:/Users/tlinnet/Documents/My Dropbox/Speciale/5NT-project/Mutant-construct/predict_reactivity/propka
### If you have the script in your working directory the
#run ./propka.py
### You can also make the script general available. Put it into your python path. Ex: C:\Program Files (x86)\PyMOL\PyMOL\modules Then do instead:
import propka
### The fastest method is just to write propka. Then the last pymol molecule is assumed and send to server. verbose=yes makes the script gossip mode.
fetch 4ins, async=0
propka
#fetch 1hp1, async=0
#propka logtime=_, resi=5-10.20-30, resn=CYS.ATP.TRP, verbose=yes
### Fetch 4ins from web. async make sure, we dont execute script before molecule is loaded. The resi and resn prints the interesting results right to command line.
#fetch 4ins, async=0
#propka chain=*, resi=5-10.20-30, resn=ASP.CYS, logtime=_
### If there is no web connection, one can process a local .pka file. Either from a previous run or from a downloaded propka webpage result.
### Then run and point to .pka file with: pkafile=./Results_propka/pkafile.pka Remember the dot "." in the start, to make it start in the current directory.
#load 4ins.pdb
#propka pkafile=./Results_propka/4ins_.pka, resi=18.25-30, resn=cys,
### Some more examples. This molecule has 550 residues, so takes a longer time. We select to run the last molecule, by writing: molecule=1hp1
#fetch 4ins, async=0
#fetch 1hp1, async=0
#propka molecule=1hp1, chain=A, resi=300-308.513, resn=CYS.ATP.TRP, logtime=_, verbose=no, showresult=no
#propka molecule=1hp1, pkafile=./Results_propka/1hp1_.pka, verbose=yes
Input paramaters
############################################Input parameters: propka############################################
############# The order of input and changable things:
propka(molecule="NIL",chain="*",resi="0",resn="NIL",method="upload",logtime=time.strftime("%m%d",time.localtime()),server_wait=3.0,version="v3.1",verbose="no",showresult="no",pkafile="NIL")
# method : method=upload is default. This sends .pdb file and request result from propka server.
## method=file will only process a manual .pka file, and write a pymol command file. No use of mechanize.
## If one points to an local .pka file, then method is auto-changed to method=file. This is handsome in off-line environment, ex. teaching or seminar.
# pkafile: Write the path to .pka file. Ex: pkafile=./Results_propka/4ins_.pka
# molecule : name of the molecule. Ending of file is assumed to be .pdb
# chain : which chains are saved to file, before molecule file is send to server. Separate with "." Ex: chain=A.b
# resi : Select by residue number, which residues should be printed to screen and saved to the log file: /Results_propka/_Results.log.
## Separate with "." or make ranges with "-". Ex: resi=35.40-50
# resn : Select by residue name, which residues should be printed to screen and saved to the log file: /Results_propka/_Results.log.
## Separate with "." Ex: resn=cys.tyr
# logtime : Each execution give a set of files with the job id=logtime. If logtime is not provided, the current time is used.
## Normal it usefull to set it empty. Ex: logtime=_
# verbose : Verbose is switch, to turn on messages for the mechanize section. This is handsome to see how mechanize works, and for error searching.
# showresult : Switch, to turn on all results in pymol command window. Ex: showresult=yes
# server_wait=10.0 is default. This defines how long time between asking the server for a result. Set no lower than 3 seconds.
# version=v3.1 is default. This is what version of propka which would be used.
## Possible: 'v3.1','v3.0','v2.0'. If a newer version is available than the current v3.1, a error message is raised to make user update the script.
############################################Input parameters: getpropka############################################
############# The order of input and changable things:
getpropka(PDB="NIL",chain="*",resi="0",resn="NIL",source="upload",PDBID="",logtime=time.strftime("%Y%m%d%H%M%S",time.localtime()),server_wait=3.0,version="v3.1",verbose="no",showresult="no")
# PDB: points the path to a .pdb file. This is auto-set from propka function.
# source : source=upload is default and is set at the propka webpage.
# source=ID, PDBID=4ake , one can print to the command line, the pka value for any official pdb ID. No files are displayed in pymol.
# PDBID: is used as the 4 number/letter pdb code, when invoking source=ID.
Mutagenesis analysis
This script was developed with the intention of making analysis of possible mutants easier. For example, the reactivity of Cysteines in FRET maleimide labelling is determined by the fraction of the Cysteine residue which is negatively charged (C-). This fraction is related to its pKa value and the pH of the buffer: f(C-)=1/(10(pK-pH)+1). So, one would be interested in having the lowest possible pKa value as possible. Ideally lower than the pH of the buffer. To analyse where to make the best mutant in your protein, you could do the following for several residues. We do the mutagenesis in the command line, since we then could loop over the residues in the protein.
fetch 1ohr, async=0
create 1ohrB3C, 1ohr
hide everything, all
show cartoon, 1ohrB3C
cmd.wizard("mutagenesis")
cmd.do("refresh_wizard")
# To get an overview over the wizard API:
for i in dir(cmd.get_wizard()): print i
# lets mutate chain B residue 3 to CYS. (1ohrB3C)
cmd.get_wizard().set_mode("CYS")
cmd.get_wizard().do_select("/1ohrB3C//B/3")
# Select the first rotamer, which is most probable
cmd.frame(1)
# Apply the mutation
cmd.get_wizard().apply()
# Close wizard
cmd.set_wizard("done")
#OR cmd.wizard(None)
import propka
propka resi=3
zoom /1ohrB3C//B/3
So, in a loop with defined residues, this could look like following code. Note, now we are quite happy for the result log file, since it collects the pka for the proteins.
fetch 1ohr, async=0
import propka
hide everything, all
### We make it in python blocks, so pymol don't speed ahead.
python
resis = [["A",3],["B",6]]
for resi in resis:
newname="1ohr%s%sC"%(resi[0],resi[1])
cmd.create(newname,"1ohr")
cmd.show("cartoon","1ohr%s%sC"%(resi[0],resi[1]))
cmd.wizard("mutagenesis")
cmd.do("refresh_wizard")
cmd.get_wizard().set_mode("CYS")
selection="/%s//%s/%s"%(newname,resi[0],resi[1])
cmd.get_wizard().do_select(selection)
cmd.frame(1)
cmd.get_wizard().apply()
cmd.set_wizard("done")
# When we import a module in python, the namespace is normally: module.function
# And we see, that propka expect resi to be in "str" format.
# And we don't want the logtime function
propka.propka(resi="%s"%resi[1],logtime="")
selection="/%s//%s/%s"%(newname,resi[0],resi[1])
cmd.select("Mutation%s%s"%(resi[0],resi[1]),"byres %s"%(selection))
print resi
python end
A little warning though. You need to be carefull about the rotamer you're choosing. It can happen that the first rotamer ends up being in physically non-reasonable contact distance to other residues, so atoms become overlayed. Also, the mutagenesis wizard can have the funny habit of sometimes not adding hydrogens to terminal -C or -N after mutating a residue.
Python Code
The code can be downloaded fast from here http://tinyurl.com/pymolpropka.
- wget http://tinyurl.com/pymolpropka
- mv pymolpropka propka.py
#-------------------------------------------------------------------------------
# Name: propka for pymol
# Purpose: To fetch and display the pka values for protein of intetest
#
# Author: Troels E. Linnet
#
# Created: 14/08/2011
# Copyright: (c) Troels E. Linnet 2011
# Contact: tlinnet snabela gmail dot com
# Licence: Free for all
#
# Download: http://tinyurl.com/pymolpropka
#
#-------------------------------------------------------------------------------
"""
The PROPKA method is developed by the
Jensen Research Group
Department of Chemistry
University of Copenhagen
Please cite these references in publications:
Hui Li, Andrew D. Robertson, and Jan H. Jensen
"Very Fast Empirical Prediction and Interpretation of Protein pKa Values"
Proteins, 2005, 61, 704-721.
Delphine C. Bas, David M. Rogers, and Jan H. Jensen
"Very Fast Prediction and Rationalization of pKa Values for Protein-Ligand Complexes"
Proteins, 2008, 73, 765-783.
Mats H.M. Olsson, Chresten R. Soendergard, Michal Rostkowski, and Jan H. Jensen
"PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa predictions"
Journal of Chemical Theory and Computation, 2011 7 (2), 525-537
Chresten R. Soendergaard, Mats H.M. Olsson, Michaz Rostkowski, and Jan H. Jensen
"Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values"
Journal of Chemical Theory and Computation, 2011 in press
"""
#-------------------------------------------------------------------------------
# The script needs mechanize to run.
# On windows, it is not easy to make additional modules available for pymol. So put in into your working folder.
#1)The easy manual way:
#a)Go to: http://wwwsearch.sourceforge.net/mechanize/download.html
#b)Download mechanize-0.2.5.zip. http://pypi.python.org/packages/source/m/mechanize/mechanize-0.2.5.zip
#c)Extract to .\mechanize-0.2.5 then move the in-side folder "mechanize" to your folder with propka.py. The rest of .\mechanize-0.2.5 you don't need.
#You can also see other places where you could put the "mechanize" folder. Write this in pymol to see the paths where pymol is searching for "mechanize"
# import sys; print(sys.path)
#-------------------------------------------------------------------------------
"""
Example for pymol script to start the functions. For example: trypropka.pml
Execute with pymol or start pymol and: File->Run->trypropka.pml
##############################################################################################################################################################################################################################
### Point to your directory with your pdb file and where to save the results
#cd /homes/linnet/Documents/Speciale/5NT-project/Mutant-construct/predict_reactivity/propka
cd C:/Users/tlinnet/Documents/My Dropbox/Speciale/5NT-project/Mutant-construct/predict_reactivity/propka
### If you have the script in your working directory the
#run ./propka.py
### You can also make the script general available. Put it into your python path. Ex: C:\Program Files (x86)\PyMOL\PyMOL\modules Then do instead:
import propka
### The fastest method is just to write propka. Then the last pymol molecule is assumed and send to server. verbose=yes makes the script gossip mode.
fetch 4ins, async=0
propka
#fetch 1hp1, async=0
#propka logtime=_, resi=5-10.20-30, resn=CYS.ATP.TRP, verbose=yes
### Fetch 4ins from web. async make sure, we dont execute script before molecule is loaded. The resi and resn prints the interesting results right to command line.
#fetch 4ins, async=0
#propka chain=*, resi=5-10.20-30, resn=ASP.CYS, logtime=_
### If there is no web connection, one can process a local .pka file. Either from a previous run or from a downloaded propka webpage result.
### Then run and point to .pka file with: pkafile=./Results_propka/pkafile.pka Remember the dot "." in the start, to make it start in the current directory.
#load 4ins.pdb
#propka pkafile=./Results_propka/4ins_.pka, resi=18.25-30, resn=cys,
### Some more examples. This molecule has 550 residues, so takes a longer time. We select to run the last molecule, by writing: molecule=1hp1
#fetch 4ins, async=0
#fetch 1hp1, async=0
#propka molecule=1hp1, chain=A, resi=300-308.513, resn=CYS.ATP.TRP, logtime=_, verbose=no, showresult=no
#propka molecule=1hp1, pkafile=./Results_propka/1hp1_.pka, verbose=yes
### One can also just make a lookup for a protein. Use function: getpropka
### Note. This does only print the result to the pymol command line
#getpropka source=ID, PDBID=4ake, logtime=_, showresult=yes
#getpropka source=ID, PDBID=4ins, logtime=_, server_wait=10.0, verbose=yes, showresult=no
############################################Input parameters: propka############################################
############# The order of input and changable things:
############# propka(molecule="NIL",chain="*",resi="0",resn="NIL",method="upload",logtime=time.strftime("%m%d",time.localtime()),server_wait=3.0,version="v3.1",verbose="no",showresult="no",pkafile="NIL")
# method : method=upload is default. This sends .pdb file and request result from propka server.
## method=file will only process a manual .pka file, and write a pymol command file. No use of mechanize.
## If one points to an local .pka file, then method is auto-changed to method=file. This is handsome in off-line environment, ex. teaching or seminar.
# pkafile: Write the path to .pka file. Ex: pkafile=./Results_propka/4ins_.pka
# molecule : name of the molecule. Ending of file is assumed to be .pdb
# chain : which chains are saved to file, before molecule file is send to server. Separate with "." Ex: chain=A.b
# resi : Select by residue number, which residues should be printed to screen and saved to the log file: /Results_propka/_Results.log.
## Separate with "." or make ranges with "-". Ex: resi=35.40-50
# resn : Select by residue name, which residues should be printed to screen and saved to the log file: /Results_propka/_Results.log.
## Separate with "." Ex: resn=cys.tyr
# logtime : Each execution give a set of files with the job id=logtime. If logtime is not provided, the current time is used.
## Normal it usefull to set it empty. Ex: logtime=_
# verbose : Verbose is switch, to turn on messages for the mechanize section. This is handsome to see how mechanize works, and for error searching.
# showresult : Switch, to turn on all results in pymol command window. Ex: showresult=yes
# server_wait=10.0 is default. This defines how long time between asking the server for a result. Set no lower than 3 seconds.
# version=v3.1 is default. This is what version of propka which would be used.
## Possible: 'v3.1','v3.0','v2.0'. If a newer version is available than the current v3.1, a error message is raised to make user update the script.
############################################Input parameters: getpropka############################################
############# The order of input and changable things:
############# getpropka(PDB="NIL",chain="*",resi="0",resn="NIL",source="upload",PDBID="",logtime=time.strftime("%Y%m%d%H%M%S",time.localtime()),server_wait=3.0,version="v3.1",verbose="no",showresult="no")
# PDB: points the path to a .pdb file. This is auto-set from propka function.
# source : source=upload is default and is set at the propka webpage.
# source=ID, PDBID=4ake , one can print to the command line, the pka value for any official pdb ID. No files are displayed in pymol.
# PDBID: is used as the 4 number/letter pdb code, when invoking source=ID.
##############################################################################################################################################################################################################################
"""
try: from pymol import cmd; runningpymol='yes'
except: runningpymol='no'; pass
import time, platform, os
def propka(molecule="NIL",chain="*",resi="0",resn="NIL",method="upload",logtime=time.strftime("%m%d",time.localtime()),server_wait=3.0,version="v3.1",verbose="no",showresult="no",pkafile="NIL"):
Script_Version="20110823"
### First we have to be sure, we give reasonable arguments
if pkafile!="NIL":
method='file'
assert method in ['upload', 'file'], "'method' has to be either: method=upload or method=file"
### If molecule="all", then try to get the last molecule
##assert molecule not in ['NIL'], "You always have to provide molecule name. Example: molecule=4ins"
if molecule=="NIL":
assert len(cmd.get_names())!=0, "Did you forget to load a molecule? There are no objects in pymol."
molecule=cmd.get_names()[-1]
### To print out to screen for selected residues. Can be separated with "." or make ranges with "-". Example: resi="4-8.10"
if resi != "0": resi_range = ResiRange(resi)
else: resi_range=[]
### Also works for residue names. They are all converted to bigger letters. Example: resn="cys.Tyr"
if resn != "NIL": resn_range = ResnRange(resn)
else: resn_range = resn
### Make chain range, and upper case.
chain = ChainRange(chain)
### Make result directory. We also the absolut path to the new directory.
Newdir = createdirs()
if method=="upload":
### We try to load mechanize. If this fail, one can always get the .pka file manual and the run: method=file
try: import mechanize; importedmechanize='yes'
except ImportError: print("Import error. Is a module missing?"); print(sys.exc_info()); print("Look if missing module is in your python path\n%s")%sys.path;importedmechanize='no'; import mechanize
### The name for the new molecule
newmolecule = "%s%s"%(molecule,logtime)
### Create the new molecule from original loaded and for the specified chains. Save it, and disable the old molecule.
cmd.create("%s"%newmolecule, "%s and chain %s"%(molecule,chain))
cmd.save("%s%s.pdb"%(Newdir,newmolecule), "%s"%newmolecule)
cmd.disable("%s"%molecule)
if molecule=="all": cmd.enable("%s"%molecule); cmd.show("cartoon", "%s"%molecule)
### Let the new molecule be shown in cartoon.
cmd.hide("everything", "%s"%newmolecule)
cmd.show("cartoon", "%s"%newmolecule)
### Make the absolut path to the newly created .pdb file.
PDB="%s%s.pdb"%(Newdir,newmolecule);source="upload"; PDBID=""
### Request server, and get the absolut path to the result file.
pkafile = getpropka(PDB,chain,resi,resn,source,PDBID,logtime,server_wait,version,verbose,showresult)
### Open the result file and put in into a handy list.
list_results,ligands_results = importpropkaresult(pkafile)
### Now we check if the script is actually the newest one.
Web_Version,Script_Version=checkversion(Script_Version,verbose)
if float(Web_Version) > float(Script_Version):
print('\n\n####################################\nWarning: The author has updated the pymol propka script.\nPresent: %s > Script: %s \nThe new script is available at "http://pymolwiki.org/index.php/Propka" or "http://tinyurl.com/pymolpropka"\n####################################\n\n'%(Web_Version,Script_Version))
if method=="file":
assert pkafile not in ['NIL'], "You have to provide path to file. Example: pkafile=./Results_propka/4ins_2011.pka"
assert ".pka" in pkafile, 'The propka result file should end with ".pka" \nExample: pkafile=./Results_propka/4ins_2011.pka \npkafile=%s'%(pkafile)
### The name for the molecule we pass to the writing script of pymol commands
newmolecule = "%s"%molecule
cmd.hide("everything", "%s"%newmolecule)
cmd.show("cartoon", "%s"%newmolecule)
### We open the result file we have got in the manual way and put in into a handy list.
list_results,ligands_results = importpropkaresult(pkafile)
### Then we print the interesting residues to the screen.
printpropkaresult(list_results, resi, resi_range, resn, resn_range, showresult, ligands_results)
### Now create the pymol command file. This should label the protein. We get back the absolut path to the file, so we can execute it.
result_pka_pymol_name = writepymolcmd(newmolecule,pkafile,verbose)
### Now run our command file. But only if we are running pymol.
if runningpymol=='yes': cmd.do("run %s"%result_pka_pymol_name)
##if runningpymol=='yes': cmd.do("@%s"%result_pka_pymol_name)
return(list_results)
if runningpymol !='no': cmd.extend("propka",propka)
def getpropka(PDB="NIL",chain="*",resi="0",resn="NIL",source="upload",PDBID="",logtime=time.strftime("%Y%m%d%H%M%S",time.localtime()),server_wait=3.0,version="v3.1",verbose="no",showresult="no"):
try: import mechanize; importedmechanize='yes'
except ImportError: print("Import error. Is a module missing?"); print(sys.exc_info()); print("Look if missing module is in your python path \n %s"%sys.path);importedmechanize='no'
propka_v_201108 = 3.1
url = "http://propka.ki.ku.dk/"
assert version in ['v2.0', 'v3.0', 'v3.1'], "'version' has to be either: 'v2.0', 'v3.0', 'v3.1'"
assert source in ['ID', 'upload', 'addr', 'input_file'], "'source' has to be either: 'ID', 'upload', 'addr', 'input_file'"
if source=="upload": assert PDB not in ['NIL'], "You always have to provide PDB path. Example: PDB=.\Results_propka\4ins2011.pdb"
if source=="ID": assert len(PDBID)==4 , "PDBID has to be 4 characters"
### To print out to screen for selected residues. Can be separated with "." or make ranges with "-". Example: resi="4-8.10"
if resi != "0": resi_range = ResiRange(resi)
else: resi_range=[]
### Also works for residue names. They are all converted to bigger letters. Example: resn="cys.Tyr"
if resn != "NIL": resn_range = ResnRange(resn)
else: resn_range = resn
### Start the browser
br = mechanize.Browser()
### We pass to the server, that we are not a browser, but this python script. Can be used for statistics at the propka server.
br.addheaders = [('User-agent', 'pythonMechanizeClient')]
### To turn on debugging messages
##br.set_debug_http(True)
### To open the start page.
page_start = br.open(url)
read_start = page_start.read()
if verbose == 'yes': print(br.title()); print(br.geturl())
### To get available forms
page_forms = [f.name for f in br.forms()]
if verbose == 'yes': print(page_forms)
### Select first form
br.select_form(name=page_forms[0])
## Print the current selected form, so we see that we values we start with.
if verbose == 'yes': print(br.form)
### Print the parameters of the 'version' RadioControl button and current value
if verbose == 'yes': print(br.find_control(name='version')), br.find_control(name='version').value
### This is to check, that the current script is "up-to-date".
propka_v_present = float(br.find_control(name='version').value[0].replace('v',''))
if propka_v_present > propka_v_201108:
raise UserWarning('\nNew version of propka exist.\nCheck/Update your script.\nPresent:v%s > Script:v%s'%(propka_v_present,propka_v_201108))
### Change the parameters of the 'version' radio button and then reprint the new value. Input has to be in a list [input].
br.form['version'] = [version]
if verbose == 'yes': print(br.find_control(name='version').value)
### Print the parameters of the 'source' RadioControl button and current value
if verbose == 'yes': print(br.find_control(name='source'), br.find_control(name='source').value)
### Change the parameters of the 'source' radio button and then reprint the new value. Input has to be in a list [input].
br.form['source'] = [source]
if verbose == 'yes': print(br.find_control(name='source').value)
### This step was the must strange and took a long time. For finding the information and the double negative way.
### One have to enable the pdb button. Read more here: http://wwwsearch.sourceforge.net/old/ClientForm/ ("# All Controls may be disabled.....)
PDBID_control = br.find_control("PDBID")
PDB_control = br.find_control("PDB")
if verbose == 'yes': print(PDBID_control.disabled, PDB_control.disabled)
if source == "ID": PDBID_control.disabled=False; PDB_control.disabled=True
if source == "upload": PDBID_control.disabled=True; PDB_control.disabled=False
if verbose == 'yes': print(PDBID_control.disabled, PDB_control.disabled)
### We create the result dir, and take with us the 'path' to the result dir.
Newdir = createdirs()
### Open all the files, and assign them.
if source == "upload": filename = PDB
if source == "ID": filename = PDBID
files = openfiles(Newdir, filename, logtime, source)
result_pka_file=files[0];result_input_pka_file=files[1];result_log=files[2];filepath=files[3];result_pka_pkafile=files[4];result_pka_file_stripped=files[5];result_pka_file_bonds=files[6]
## Print the parameters of the 'PDBID' TextControl button and current value
if source == "ID" and verbose == 'yes': print(br.find_control(name='PDBID')); print(br.find_control(name='PDBID').value)
## Change the parameters of the 'PDBID' TextControl and then reprint the new value. Input has just to be a string.
if source == "ID": br.form["PDBID"] = PDBID
if source == "ID" and verbose == 'yes': print(br.find_control(name='PDBID').value)
## Print the parameters of the 'PDB' TextControl button and current value
if source == "upload" and verbose == 'yes': print(br.find_control(name='PDB')); print(br.find_control(name='PDB').value)
## Change the parameters of the 'PDB' FileControl and then reprint the new value. Input has just to be a string.
if source == "upload": PDBfilename=PDB; PDBfilenamepath=PDB
if source == "upload": br.form.add_file(open(PDBfilename), 'text/plain', PDBfilenamepath, name='PDB')
if source == "upload" and verbose == 'yes': print(br.find_control(name='PDB')); print(br.find_control(name='PDB').value)
## Now reprint the current selected form, so we see that we have the right values.
if verbose == 'yes': print(br.form)
### Make "how" we would like the next request. We would like to "Click the submit button", but we have not opened the request yet.
req = br.click(type="submit", nr=0)
### Have to pass by a mechanize exception. Thats the reason for the why True
### The error was due to: br.open(req)
###### mechanize._response.httperror_seek_wrapper: HTTP Error refresh: The HTTP server returned a redirect error that would lead to an infinite loop.
###### The last 30x error message was:
###### OK
### I haven't been able to find the refresh problem or extend the time. So we make a pass on the raised exception.
try:
print("Now sending request to server")
br.open(req)
### If there is raised an exception, we jump through to the result page after some sleep.
except mechanize.HTTPError:
### We can extract the jobid from the current browser url.
jobid = br.geturl()[32:-5]
### We notice how the script at the server presents the final result page.
url_result = url + "pka/" + jobid + ".html"
### Now we continue to try to find the result page, until we have succes. If page doesn't exist, we wait a little.
while True:
print("Result still not there. Waiting %s seconds more"%server_wait)
time.sleep(float(server_wait))
### To pass the "break" after the exception, we make a hack, wait and then go to the result page, which is the jobid.
try:
page_result = br.open(url_result)
read_result = page_result.read()
### If we don't receive a error in getting the result page, we break out of the while loop.
break
### If the page doesn't exist yet. We go back in the while loop.
except mechanize.HTTPError:
### Wait another round
pass
### If we get a timeout, we also wait.
except mechanize.URLError:
### Wait another round
pass
htmlresult="The detailed result is now available at: %s"%br.geturl()
print(htmlresult)
read_result = br.response().read()
## Now save the available links from the current page. But only links that satisfy the expression.
links_result = []
for l in br.links(url_regex='http://propka.ki.ku.dk/pka'):
links_result.append(l)
## We also extract the information for neighbour bons. This is given in the url links.
bonds=[]
for l in br.links(url_regex='http://propka.ki.ku.dk/view/new_view.cgi'):
l_split=str(l).split()
lresn=l_split[2]
lresi=l_split[3]
lchain=l_split[4]
lurl=l_split[1]
lurl_split=lurl.split("&")
lresn2=lurl_split[1]
lchain2=lurl_split[2]
lpka=lurl_split[3]
ldesolvation=lurl_split[4]
lneighbours=lurl_split[5:]
for i in range(len(lneighbours)):
bonds.append([lresn,lresi,lchain,lresn2,lchain2,lpka,ldesolvation,lneighbours[i]])
### Now follow the link to the .propka_input resultpage
if len(links_result) > 1: br.follow_link(links_result[1])
### Now get the page text for the current link
if len(links_result) > 1: read_result1 = br.response().read()
### Save the result
if len(links_result) > 1: result_input_pka_file.write(read_result1)
### Now follow the link to the .pka resultpage
if len(links_result) > 1: br.back()
result_input_pka_file.close()
### Now follow first link. "Should be" available for all versions of propka.
br.follow_link(links_result[0])
### Now get the page for the current link
read_result0 = br.response().read()
### Save the result and close file.
result_pka_file.write(read_result0)
result_pka_file.close()
### Now get the result in a list, which is sorted
list_results,ligands_results = importpropkaresult(result_pka_pkafile)
### Print to log file
result_log.write("# executed: %s \n# logtime: %s \n# source=%s \n# PDB=%s \n# chain=%s \n# PDBID=%s \n# server_wait=%s version=%s verbose=%s showresult=%s \n# resi=%s resn=%s\n# %s \n"%(time.strftime("%Y%m%d%H%M%S",time.localtime()),logtime,source,PDB,chain,PDBID,server_wait,version,verbose,showresult,resi,resn,htmlresult))
### Print to screen
printpropkaresult(list_results, resi, resi_range, resn, resn_range, showresult, ligands_results)
### Now write to log and the stripped file
for l in list_results:
if resi != "0" and int(l[1]) in resi_range:
result_log.write("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]) + '\n')
if resn != "NIL" and l[0] in resn_range and int(l[1]) not in resi_range:
result_log.write("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]) + '\n')
result_pka_file_stripped.write("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]) + '\n')
for l in ligands_results:
if resn != "NIL" and l[0] in resn_range:
result_log.write("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]) + '\n')
result_pka_file_stripped.write("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]) + '\n')
result_pka_file_stripped.close()
result_log.close()
### Now handle the bonds. We have to delete dublicates first.
bonds.sort()
last=bonds[-1]
for i in range(len(bonds)-2, -1, -1):
if last == bonds[i]: del bonds[i]
else: last=bonds[i]
### Now make a selection for known residue
bonds_selected=[]
bonds_ligands=[]
for l in bonds:
if l[0][6:] in ['ASP', 'GLU', 'ARG', 'LYS', 'HIS', 'CYS', 'TYR', 'C-', 'N+']:
bonds_selected.append(l)
else:
bonds_ligands.append(l)
### And now sort it.
bonds_selected.sort(key=lambda residue: int(residue[1]))
### Now write it to file
bonddic={'=':' ',':':' ',',':' ',"'":" "}
for l in bonds_selected:
nb = replace_all(l[7],bonddic)
result_pka_file_bonds.write("%3s %3s %s %7s %7s %9s %17s %s"%(l[0][6:],l[1],l[2][:1],l[3][8:],l[4],l[5],l[6],nb) + '\n')
for l in bonds_ligands:
nb = replace_all(l[7],bonddic)
result_pka_file_bonds.write("%3s %3s %s %7s %7s %9s %17s %s"%(l[0][6:],l[1],l[2][:1],l[3][8:],l[4],l[5],l[6],nb) + '\n')
result_pka_file_bonds.close()
return(result_pka_pkafile)
if runningpymol !='no': cmd.extend("getpropka",getpropka)
def openpymolfiles(pkafile):
result_pka_pymol_name = pkafile.replace(".pka",".pml")
result_pka_pymol = open(result_pka_pymol_name, "w")
return(result_pka_pymol, result_pka_pymol_name)
def printpropkaresult(list_results, resi, resi_range, resn, resn_range, showresult, ligands_results):
for l in list_results:
if resi != "0" and int(l[1]) in resi_range:
if showresult != 'yes': print("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]))
if resn != "NIL" and l[0] in resn_range and int(l[1]) not in resi_range:
if showresult != 'yes': print("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]))
if showresult == 'yes': print("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]))
for l in ligands_results:
if resn != "NIL" and l[0] in resn_range:
if showresult != 'yes': print("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]))
if showresult == 'yes': print("%3s %3s %s %6s %3s %5s %3s %4s %s"%(l[0],l[1],l[2],l[3],l[4],l[5],l[6],l[7],l[8]))
def importpropkaresult(result_pka_pkafile):
result_pka_file = open(result_pka_pkafile, "r")
list_results = []
ligands_results = []
##bonding_partners = []
for l in result_pka_file:
if not l.strip():
continue
else:
### To search for the right lines
if l.strip().split()[0] in ['ASP', 'GLU', 'ARG', 'LYS', 'HIS', 'CYS', 'TYR', 'C-', 'N+'] and len(l.strip().split())>20:
list_results.append([l.strip().split()[0], l.strip().split()[1], l.strip().split()[2], l.strip().split()[3], l.strip().split()[4], l.strip().split()[6], l.strip().split()[7], l.strip().split()[8], l.strip().split()[9]])
##bonding_partners.append(l.strip().split()[11]);bonding_partners.append(l.strip().split()[15]);bonding_partners.append(l.strip().split()[19])
if l.strip().split()[0] not in ['ASP', 'GLU', 'ARG', 'LYS', 'HIS', 'CYS', 'TYR', 'C-', 'N+'] and len(l.strip().split())>20:
ligands_results.append([l.strip().split()[0], l.strip().split()[1], l.strip().split()[2], l.strip().split()[3], l.strip().split()[4], l.strip().split()[6], l.strip().split()[7], l.strip().split()[8], l.strip().split()[9]])
##bonding_partners.append(l.strip().split()[11]);bonding_partners.append(l.strip().split()[15]);bonding_partners.append(l.strip().split()[19])
### Sort the result after the residue number and then chain.
list_results.sort(key=lambda residue: int(residue[1]))
list_results.sort(key=lambda chain: chain[2])
##bonding_partners=uniqifi(bonding_partners)
##bonding_partners[:] = [x for x in bonding_partners if x != "XXX"]
result_pka_file.close()
return(list_results,ligands_results)
def importpropkabonds(result_pka_pkafile):
bonds=[]
result_pka_file_bonds=open(result_pka_pkafile[:-4]+".bonds", "r")
for l in result_pka_file_bonds:
bonds.append(l.split())
result_pka_file_bonds.close()
return(bonds)
def createdirs():
if platform.system() == 'Windows': Newdir = os.getcwd()+"\Results_propka\\"
if platform.system() == 'Linux': Newdir = os.getcwd()+"/Results_propka/"
if not os.path.exists(Newdir): os.makedirs(Newdir)
return(Newdir)
def openfiles(Newdir, filename, logtime, source):
if source == "upload":
result_pka_pkafile = filename.replace(".pdb",".pka")
result_pka_input_pkafile = filename.replace(".pdb",".propka_input")
result_log_name = "%s_Results.log"%(Newdir)
result_pka_file_stripped_name = filename.replace(".pdb",".stripped")
result_pka_file_bonds_name = filename.replace(".pdb",".bonds")
if source == "ID":
result_pka_pkafile = "%s%s%s.pka"%(Newdir,filename,logtime)
result_pka_input_pkafile = "%s%s%s.propka_input"%(Newdir,filename,logtime)
result_log_name = "%s_Results.log"%(Newdir)
result_pka_file_stripped_name = "%s%s%s.stripped"%(Newdir,filename,logtime)
result_pka_file_bonds_name = "%s%s%s.bonds"%(Newdir,filename,logtime)
if platform.system() == 'Windows': filepath = "\\"
if platform.system() == 'Linux': filepath = "/"
### Open the files
result_pka_file = open(result_pka_pkafile, "w")
result_input_pka_file = open(result_pka_input_pkafile, "w")
result_log = open(result_log_name, "a")
result_pka_file_stripped = open(result_pka_file_stripped_name, "w")
result_pka_file_bonds = open(result_pka_file_bonds_name, "w")
return(result_pka_file, result_input_pka_file, result_log, filepath, result_pka_pkafile,result_pka_file_stripped,result_pka_file_bonds)
def ResiRange(resi):
resi = resi.split('.')
resiList = []
for i in resi:
if '-' in i:
tmp = i.split('-')
resiList.extend(range(int(tmp[0]),int(tmp[-1])+1))
if '-' not in i:
resiList.append(int(i))
return(resiList)
def ResnRange(resn):
resn_split = resn.split('.')
resn_range = [resnr.upper() for resnr in resn_split]
return(resn_range)
def ChainRange(chain):
chainstring = chain.replace(".","+").upper()
return(chainstring)
def writepymolcmd(newmolecule,pkafile,verbose):
list_results,ligands_results = importpropkaresult(pkafile)
### Now find the available bonding partners that pymol knows of
bonding_partners = []
bonding_partners_str = cmd.get_pdbstr("%s and resn * and not resn ASP+GLU+ARG+LYS+HIS+CYS+TYR+GLN+ASN+SER+THR+GLY+PHE+LEU+ALA+ILE+TRP+MET+PRO+VAL+HOH"%(newmolecule))
for i in range(len(bonding_partners_str.splitlines())-1):
bonding_partners_split = bonding_partners_str.splitlines()[i].split()
if bonding_partners_split[0] == "HETATM" or bonding_partners_split[0] == "ATOM" :
bonding_partners_single = bonding_partners_split[3]
bonding_partners.append(bonding_partners_single)
bonding_partners=uniqifi(bonding_partners)
if verbose == 'yes': print("And other possible bonding partners is: %s"%(bonding_partners))
### Read in the bond file, if it exists
makebonds = "no"
if os.path.isfile(pkafile[:-4]+".bonds"): bonds = importpropkabonds(pkafile); makebonds = "yes"
### Open the pymol command file for writing
files_pka_pymol = openpymolfiles(pkafile)
result_pka_pymol=files_pka_pymol[0];result_pka_pymol_name=files_pka_pymol[1]
### Make some dictionary for propka->pymol name conversion
dictio = {'ASP':'CG', 'GLU':'CD', 'ARG':'CZ', 'LYS':'NZ', 'HIS':'CG', 'CYS':'SG', 'TYR':'OH', 'C-':'C', 'N+':'N','NTR':'N','CTR':'C','GLN':'CD','ASN':'CG','SER':'OG','THR':'OG1','GLY':'CA','PHE':'CZ','LEU':'CG','ALA':'CB','ILE':'CD1','TRP':'NE1','MET':'SD','PRO':'CG','VAL':'CB'}
dictio2 = {'ASP':'D', 'GLU':'E', 'ARG':'R', 'LYS':'K', 'HIS':'H', 'CYS':'C', 'TYR':'Y', 'C-':'C-', 'N+':'N+'}
### This list is from: http://en.wikipedia.org/wiki/Protein_pKa_calculations
pkaaminoacid=['ASP','GLU','ARG','LYS','HIS','CYS','TYR']
pkadictio = {'ASP':3.9, 'GLU':4.3, 'ARG':12.0, 'LYS':10.5, 'HIS':6.0, 'CYS':8.3, 'TYR':10.1}
### Now start write to the file.
### Try to make silent
##result_pka_pymol.write("cmd.feedback('disable','all','actions')\n")
##result_pka_pymol.write("cmd.feedback('disable','all','results')\n")
### Change the GUI width, to make the long names possible.
result_pka_pymol.write("cmd.set('internal_gui_width','360')\n")
### Set fonts
result_pka_pymol.write("cmd.set('label_font_id','12')\n")
result_pka_pymol.write("cmd.set('label_size','-0.5')\n")
result_pka_pymol.write("cmd.set('label_color','grey')\n")
### No auto zoom the new objects
result_pka_pymol.write("cmd.set('auto_zoom','off')\n")
### The name for the molecules are defined here
pkamolecule="%spKa"%(newmolecule)
pkalabelmolecule="%sLab"%(newmolecule)
### Create the groups now, so they come in order. They will be empty
result_pka_pymol.write("cmd.group('%sResi','Res*')\n"%(newmolecule))
result_pka_pymol.write("cmd.group('%sLigands','Lig*')\n"%(newmolecule))
if makebonds=="yes": result_pka_pymol.write("cmd.group('%sBonds','%sBond*')\n"%(newmolecule,newmolecule))
### Create new empty pymol pka molecules. For pka atoms and its label. This is a "bucket" we where we will put in the atoms together.
result_pka_pymol.write("cmd.create('%s','None')\n"%(pkamolecule))
result_pka_pymol.write("cmd.create('%s','None')\n"%(pkalabelmolecule))
### Now make the pka atoms and alter, color and such
for l in list_results:
name=dictio[l[0]];resn=dictio2[l[0]];resi=l[1];chain=l[2];pka=l[3];buried=l[4]
if "*" in pka: pka = pka.replace("*",""); comment="*Coupled residue"
else: comment=""
if l[0] in pkaaminoacid:
pkadiff =(float(pka)-pkadictio[l[0]])
pkadiff = "(%s)"%pkadiff
if pka=="99.99": pkadiff=""
else: pkadiff=""
### Make the selection for which atom to copy
newselection = ("/%s//%s/%s/%s"%(newmolecule,chain,resi,name))
protselect = ("%sRes_%s%s%s"%(newmolecule,chain,resn,resi))
result_pka_pymol.write("cmd.select('%s','byres %s')\n"%(protselect,newselection))
result_pka_pymol.write("cmd.show('sticks','byres %s')\n"%(protselect))
### The temporary name
tempname = ("%s%s%s%s"%(pkamolecule,chain,resi,name))
tempnamelabel = ("%s%s%s%s"%(pkalabelmolecule,chain,resi,name))
tempselect= ("/%s//%s/%s"%(tempname,chain,resi))
tempselectlabel= ("/%s//%s/%s"%(tempnamelabel,chain,resi))
### Copy the atom, call it by the residue name
result_pka_pymol.write("cmd.create('%s','%s',quiet=1)\n"%(tempname,newselection))
### Alter the name and the b value of the newly created atom
result_pka_pymol.write("cmd.alter('%s','b=%s')\n"%(tempselect,pka))
result_pka_pymol.write("cmd.alter('%s','vdw=0.5')\n"%(tempselect))
result_pka_pymol.write("cmd.alter('%s','name=%s%s%s')\n"%(tempselect,'"',pka,'"'))
### Now create a fake label atom, and translate it
result_pka_pymol.write("cmd.create('%s','%s',quiet=1)\n"%(tempnamelabel,tempname))
movelabelxyz = (1.5,0,0)
result_pka_pymol.write("cmd.translate('[%s,%s,%s]','%s',camera=0)\n"%(movelabelxyz[0],movelabelxyz[1],movelabelxyz[2],tempnamelabel))
### Labelling alternate positions are not allowed, so we delete that attribute for the label atoms.
result_pka_pymol.write("cmd.alter('%s','alt=%s%s')\n"%(tempselectlabel,'"','"'))
result_pka_pymol.write("cmd.label('%s','text_type=%spka=%s%s Bu:%s%s%s%s')\n"%(tempselectlabel,'"',pka,pkadiff,buried,'%',comment,'"'))
### Now put the atoms into a bucket of atoms
result_pka_pymol.write("cmd.create('%s','%s or (%s)',quiet=1)\n"%(pkamolecule,pkamolecule,tempselect))
result_pka_pymol.write("cmd.create('%s','%s or (%s)',quiet=1)\n"%(pkalabelmolecule,pkalabelmolecule,tempselectlabel))
### Remove the temporary atoms
result_pka_pymol.write("cmd.remove('%s')\n"%(tempname))
result_pka_pymol.write("cmd.remove('%s')\n"%(tempnamelabel))
### Delete the temporary molecule/selection
result_pka_pymol.write("cmd.delete('%s')\n"%(tempname))
result_pka_pymol.write("cmd.delete('%s')\n"%(tempnamelabel))
### Group the resi together
result_pka_pymol.write("cmd.group('%sResi','%sRes*')\n"%(newmolecule,newmolecule))
for l in ligands_results:
resn=l[0];atom=l[1];chain=l[2];pka=l[3];buried=l[4]
if verbose == 'yes': print("Ligand. resn:%s atom:%s chain:%s pka:%s buried:%s"%(resn,atom,chain,pka,buried))
if Check_bonding_partners(bonding_partners, resn)[0]:
if "*" in pka: pka = pka.replace("*",""); comment="*Coupled residue"
else: comment=""
### Make the selection for which atom to copy
ligselection = ("/%s and chain %s and resn %s and name %s"%(newmolecule,chain,resn,atom))
ligselect = ("%sLig_%s%s%s"%(newmolecule,chain,resn,atom))
result_pka_pymol.write("cmd.select('%s','%s')\n"%(ligselect,ligselection))
result_pka_pymol.write("cmd.show('sticks','byres %s')\n"%(ligselect))
result_pka_pymol.write("cmd.util.cbap('byres %s')\n"%(ligselect))
### The temporary name
tempname = ("%s%s%s%s"%(pkamolecule,chain,resn,atom))
tempnamelabel = ("%s%s%s%s"%(pkalabelmolecule,chain,resn,atom))
tempselect= ("/%s and chain %s and resn %s"%(tempname,chain,resn))
tempselectlabel= ("/%s and chain %s and resn %s"%(tempnamelabel,chain,resn))
### Copy the atom, call it by the residue name
result_pka_pymol.write("cmd.create('%s','%s',quiet=1)\n"%(tempname,ligselection))
### Alter the name and the b value of the newly created atom
result_pka_pymol.write("cmd.alter('%s','b=%s')\n"%(tempselect,pka))
result_pka_pymol.write("cmd.alter('%s','vdw=0.5')\n"%(tempselect))
result_pka_pymol.write("cmd.alter('%s','name=%s%s%s')\n"%(tempselect,'"',pka,'"'))
### Now create a fake label atom, and translate it
result_pka_pymol.write("cmd.create('%s','%s',quiet=1)\n"%(tempnamelabel,tempname))
movelabelxyz = (1.5,0,0)
result_pka_pymol.write("cmd.translate('[%s,%s,%s]','%s',camera=0)\n"%(movelabelxyz[0],movelabelxyz[1],movelabelxyz[2],tempnamelabel))
### Labelling alternate positions are not allowed, so we delete that attribute for the label atoms.
result_pka_pymol.write("cmd.alter('%s','alt=%s%s')\n"%(tempselectlabel,'"','"'))
result_pka_pymol.write("cmd.label('%s','text_type=%spka=%s Bu:%s%s%s%s')\n"%(tempselectlabel,'"',pka,buried,'%',comment,'"'))
### Now put the atoms into a bucket of atoms
result_pka_pymol.write("cmd.create('%s','%s or (%s)',quiet=1)\n"%(pkamolecule,pkamolecule,tempselect))
result_pka_pymol.write("cmd.create('%s','%s or (%s)',quiet=1)\n"%(pkalabelmolecule,pkalabelmolecule,tempselectlabel))
### Remove the temporary atoms
result_pka_pymol.write("cmd.remove('%s')\n"%(tempname))
result_pka_pymol.write("cmd.remove('%s')\n"%(tempnamelabel))
### Delete the temporary molecule/selection
result_pka_pymol.write("cmd.delete('%s')\n"%(tempname))
result_pka_pymol.write("cmd.delete('%s')\n"%(tempnamelabel))
### Group the resi together
result_pka_pymol.write("cmd.group('%sLigands','%sLig*')\n"%(newmolecule,newmolecule))
### Finish the pka atoms, and show spheres
result_pka_pymol.write("cmd.show('spheres','%s')\n"%(pkamolecule))
result_pka_pymol.write("cmd.spectrum('b','red_white_blue',selection='%s',minimum='0',maximum='14')\n"%(pkamolecule))
result_pka_pymol.write("cmd.alter('%s and name 99.9','vdw=0.8')\n"%(pkamolecule))
result_pka_pymol.write("cmd.show('spheres','%s and name 99.9')\n"%(pkamolecule))
result_pka_pymol.write("cmd.color('sulfur','%s and name 99.9')\n"%(pkamolecule))
### Now we make the bonds
if makebonds=="yes":
Bondgroups=[]
naturalaminoacids = ['ASP','GLU','ARG','LYS','HIS','CYS','TYR','NTR','N+','CTR','C-','GLN','ASN','SER','THR','GLY','PHE','LEU','ALA','ILE','TRP','MET','PRO','VAL']
for l in bonds:
if l[0] in naturalaminoacids:
name=dictio[l[0]];resi=l[1];chain=l[2];desolvation=l[6][12:];pkachange=l[11];NBresi=l[8][3:];NBchain=l[9];NBbond=l[-1][:2]
if l[8][:3] in naturalaminoacids:
NBname,cutoff=BondTypeName(dictio[l[8][:3]],NBbond)
fromselection = ("/%s//%s/%s/%s"%(newmolecule,chain,resi,name))
toselection = ("/%s//%s/%s/%s"%(newmolecule,NBchain,NBresi,NBname))
if l[8][:3]=='NTR':
extind=cmd.identify("chain %s and name N"%(NBchain))[0]
toselection = ("/%s and id %s and name N"%(newmolecule,extind))
NBresi="N+"
if l[8][:3]=='CTR':
extind=cmd.identify("chain %s and name C"%(NBchain))[-1]
toselection = ("/%s and id %s and name C"%(newmolecule,extind))
NBresi="C-"
distname = ("%s_%s%s%s%s_%s_%s"%(newmolecule,chain,resi,NBchain,NBresi,NBbond,pkachange))
result_pka_pymol.write("cmd.distance('%s','%s','%s'%s)\n"%(distname,fromselection,toselection,cutoff))
result_pka_pymol.write("cmd.color('%s','%s')\n"%(SetDashColor(NBbond),distname))
##result_pka_pymol.write("cmd.disable('%s')\n"%(distname))
Bondgroups.append("%s%s"%(chain,resi))
if l[8][:3] not in naturalaminoacids and Check_bonding_partners(bonding_partners, l[8])[0]:
cutoff=""; NBresn = Check_bonding_partners(bonding_partners, l[8])[1]; NBname=l[8][len(NBresn):]+"*"
fromselection = ("/%s//%s/%s/%s"%(newmolecule,chain,resi,name))
toselection = ("/%s and chain %s and resn %s and name %s"%(newmolecule,NBchain,NBresn,NBname))
if verbose == 'yes': print("Res->Ligand: (%s) -> (%s)"%(fromselection, toselection))
result_pka_pymol.write("cmd.show('sticks','%s')\n"%(toselection))
distname = ("%s_%s%s%s_%s_%s"%(newmolecule,chain,resi,NBresn,NBbond,pkachange))
result_pka_pymol.write("cmd.distance('%s','%s','%s'%s)\n"%(distname,fromselection,toselection,cutoff))
result_pka_pymol.write("cmd.color('%s','%s')\n"%(SetDashColor(NBbond),distname))
##result_pka_pymol.write("cmd.disable('%s')\n"%(distname))
Bondgroups.append("%s%s"%(chain,resi))
if l[0] in bonding_partners:
resn=l[0];atom=l[1];chain=l[2];desolvation=l[6][12:];pkachange=l[11];NBresi=l[8][3:];NBchain=l[9];NBbond=l[-1][:2]
if not Check_bonding_partners(bonding_partners, l[8])[0]:
NBname,cutoff=BondTypeName(dictio[l[8][:3]],NBbond)
fromselection = ("/%s and chain %s and resn %s and name %s"%(newmolecule,chain,resn,atom))
toselection = ("/%s//%s/%s/%s"%(newmolecule,NBchain,NBresi,NBname))
if l[8][:3]=='NTR':
extind=cmd.identify("chain %s and name N"%(NBchain))[0]
toselection = ("/%s and id %s and name N"%(newmolecule,extind))
NBresi="N+"
if l[8][:3]=='CTR':
extind=cmd.identify("chain %s and name C"%(NBchain))[-1]
toselection = ("/%s and id %s and name C"%(newmolecule,extind))
NBresi="C-"
distname = ("%s_%s%s%s%s%s_%s_%s"%(newmolecule,chain,resn,atom,NBchain,NBresi,NBbond,pkachange))
result_pka_pymol.write("cmd.distance('%s','%s','%s'%s)\n"%(distname,fromselection,toselection,cutoff))
result_pka_pymol.write("cmd.color('%s','%s')\n"%(SetDashColor(NBbond),distname))
Bondgroups.append("%s%s%s"%(chain,resn,atom))
##result_pka_pymol.write("cmd.disable('%s')\n"%(distname))
if Check_bonding_partners(bonding_partners, l[8])[0]:
cutoff=""; NBresn = Check_bonding_partners(bonding_partners, l[8])[1]; NBname=l[8][len(NBresn):]+"*"
fromselection = ("/%s and chain %s and resn %s and name %s"%(newmolecule,chain,resn,atom))
toselection = ("/%s and chain %s and resn %s and name %s"%(newmolecule,NBchain,NBresn,NBname))
if verbose == 'yes': print("Ligand->Ligand: (%s) -> (%s)"%(fromselection, toselection))
result_pka_pymol.write("cmd.show('sticks','%s')\n"%(toselection))
distname = ("%s_%s%s%s%s_%s_%s"%(newmolecule,chain,resn,atom,NBresn,NBbond,pkachange))
result_pka_pymol.write("cmd.distance('%s','%s','%s'%s)\n"%(distname,fromselection,toselection,cutoff))
result_pka_pymol.write("cmd.color('%s','%s')\n"%(SetDashColor(NBbond),distname))
##result_pka_pymol.write("cmd.disable('%s')\n"%(distname))
Bondgroups.append("%s%s%s"%(chain,resn,atom))
Bondgroups=uniqifi(Bondgroups)
for l in Bondgroups:
result_pka_pymol.write("cmd.group('%sBonds_%s','%s_%s*')\n"%(newmolecule,l,newmolecule,l))
result_pka_pymol.write("cmd.disable('%sBonds_%s')\n"%(newmolecule,l))
result_pka_pymol.write("cmd.group('%sBonds','%sBonds_*')\n"%(newmolecule,newmolecule))
result_pka_pymol.write("cmd.set('auto_zoom','on')\n")
##result_pka_pymol.write("cmd.feedback('enable','all','actions')\n")
##result_pka_pymol.write("cmd.feedback('enable','all','results')\n")
result_pka_pymol.close()
return(result_pka_pymol_name)
def checkversion(Script_Version="0",verbose='no',url="http://pymolwiki.org/index.php/Propka#ScriptVersion"):
try: import mechanize; importedmechanize='yes'
except ImportError: print("Import error. Is a module missing?"); print(sys.exc_info()); print("Look if missing module is in your python path \n %s"%sys.path);importedmechanize='no'
### Start the browser
br = mechanize.Browser()
### We pass to the server, that we are not a browser, but this python script. Can be used for statistics for the server.
br.addheaders = [('User-agent', 'pythonMechanizeClient')]
### To open the start page.
page_start = br.open(url)
read_start = page_start.read()
if verbose == 'yes': print(br.title()); print(br.geturl())
read_start_lines = read_start.splitlines()
for l in read_start_lines:
if "Current_Version=" in l:
Web_Version=l.split("=")[-1]
if verbose == 'yes': print(Web_Version)
return(Web_Version, Script_Version)
def replace_all(text, dic):
for i, j in dic.iteritems():
text = text.replace(i, j)
return(text)
def uniqifi(seq, idfun=None):
### Order preserving
if idfun is None:
def idfun(x): return x
seen = {}
result = []
for item in seq:
marker = idfun(item)
if marker in seen: continue
seen[marker] = 1
result.append(item)
return(result)
def BondTypeName(NBname, NBbond):
if NBbond=="SH":
cutoff=""
return(NBname,cutoff)
if NBbond=="BH":
cutoff=""
return("N",cutoff)
else:
cutoff=""
return(NBname,cutoff)
def Check_bonding_partners(bonding_partners, NBname):
answer = False
for l in bonding_partners:
if l in NBname:
answer = True
NBname = l
break
else:
answer = False
return(answer,NBname)
def SetDashColor(NBbond):
if NBbond=="SH": color="brightorange"
if NBbond=="BH": color="lightorange"
if NBbond=="CC": color="red"
return(color)
ScriptVersion
Current_Version=20110823
Changelog
- 20110823
- Fixed some issues with selection algebra of Ligands.
- Now colors Ligands automatically to purple scheme.
- 20110822
- Made the naming scheme consistent, so one can work with multiple proteins, and the grouping still works.
- Bonds to N-terminal and C-terminal did not show up. Fixed.
- If one just write "propka", the "last" molecule in the pymol object list is now assumed, instead of the first. This makes mutagenesis analysis easier.
- The pka difference from assumed standard values are now also displayed. Standard values are set to: pkadictio = {'ASP':3.9, 'GLU':4.3, 'ARG':12.0, 'LYS':10.5, 'HIS':6.0, 'CYS':8.3, 'TYR':10.1}
- The menu size is made bigger, so it can fit the long names for the bonding partners. There's also a slider that you can drag using the mouse
- --it's the tiny tab between the command line and the movie controls. Drag that left or right.
- 20110821
- The resn function was made wrong, only taking the last item of list. Fixed.
- resn= can now also be print to screen/log for ligands like ATP. Ex: resn=CYS.ATP.TRP
- Ligands are now also written to the "stripped-file". "./Results_propka/PDB.stripped"
- Bonds are now also made for Ligands.
- 20110820
- If one points to a result .pka file, then "method" is automatically set to "method=file".
- Added the ability for pka values for ligands
- Bonds are now generated for the pka atoms.
- The color scheme is changed from "rainbow" to "red_white_blue". This is easier to interpret.
- CC: COULOMBIC INTERACTION. Color is red.
- SH: SIDECHAIN HYDROGEN BOND. Color is brightorange.
- BH: BACKBONE HYDROGEN BOND. Color is lightorange.
- 20110817
- If just invoking with "propka", it will select the first molecule. And now it is possible also to write. "propka all".
- Removed the "raise UserWarning" if the script is oudated. Only a warning message is printed.
- 20110816
- Made the execution of the pymol command script silent, by only using cmd.API. This will raise a Warning, which can be ignored.
- Built-in a Script version control, to inform the user if the propka script is updated on this page
- The alternate attribute for the labeling atoms are reset. It was found pymol objected altering names for atoms which had alternate positions/values.
- Reorganized the input order, which means that: molecule=all is default.
Known bugs
- Bonds can be multiplied from amino acids to Ligands like ZN or ATP. Assume the shortest bond to be correct.
- ZN: This is caused, since ZN has no ID number and when there are several in the same chain. This can be reproduced for 1HP1, bond: A41ZNCC. Here it shows to bonds, where only the shortes is correct. No fix possible.
- ATP: This is caused, since propka sometimes eats the ending of the atom name. In the .pdb file is written O3', which propka represents like O3. Therefore a wildcard "*" is generel inserted, which can cause multiple bonds to the ATP molecule. This can be reproduced for 1HP1, bond: A504ATPSH. Here multiple bonds are made to all the O3*,O2* atoms. No fix possible.
- The propka server sometimes use another naming scheme for Ligands. These Ligands will not be pka labelled or shown in pymol. The results will still downloaded to "/.Results_propka/file.pka"
- This can be reproduced with 1BJ6. Here the propka webpage predicts the pka values for the ligands: AN7,GN1,CN3,CO2,GO2, but the .pdb file names these ligands: DA,DA,DC,DG.
- Alternative configurations of a Ligand is at the moment a problem, and will not be shown. For example 1HXB and the ligand ROC.
- The script extraxt AROC and BROC from pymol, which does match with ROC in the .pka file. Try save the protein with only one configuration of the Ligand.
- >In pymol:
- fetch 1hxb, async=0
- create 1hxbA, 1hxb and not alt B
- save 1hxbA.pdb, 1hxbA
- >Quit pymol
- >Manually replace with text editor in .pdb file "AROC" with " ROC" Remember the space " "!
- >Then in pymol
- load 1hxbA.pdb
- import propka
- propka verbose=yes